Discovery of AZD4625, a Covalent Allosteric Inhibitor of the Mutant GTPase KRAS G12C .
Kettle, J.G., Bagal, S.K., Bickerton, S., Bodnarchuk, M.S., Boyd, S., Breed, J., Carbajo, R.J., Cassar, D.J., Chakraborty, A., Cosulich, S., Cumming, I., Davies, M., Davies, N.L., Eatherton, A., Evans, L., Feron, L., Fillery, S., Gleave, E.S., Goldberg, F.W., Hanson, L., Harlfinger, S., Howard, M., Howells, R., Jackson, A., Kemmitt, P., Lamont, G., Lamont, S., Lewis, H.J., Liu, L., Niedbala, M.J., Phillips, C., Polanski, R., Raubo, P., Robb, G., Robinson, D.M., Ross, S., Sanders, M.G., Tonge, M., Whiteley, R., Wilkinson, S., Yang, J., Zhang, W.(2022) J Med Chem 65: 6940-6952
- PubMed: 35471939
- DOI: https://doi.org/10.1021/acs.jmedchem.2c00369
- Primary Citation of Related Structures:
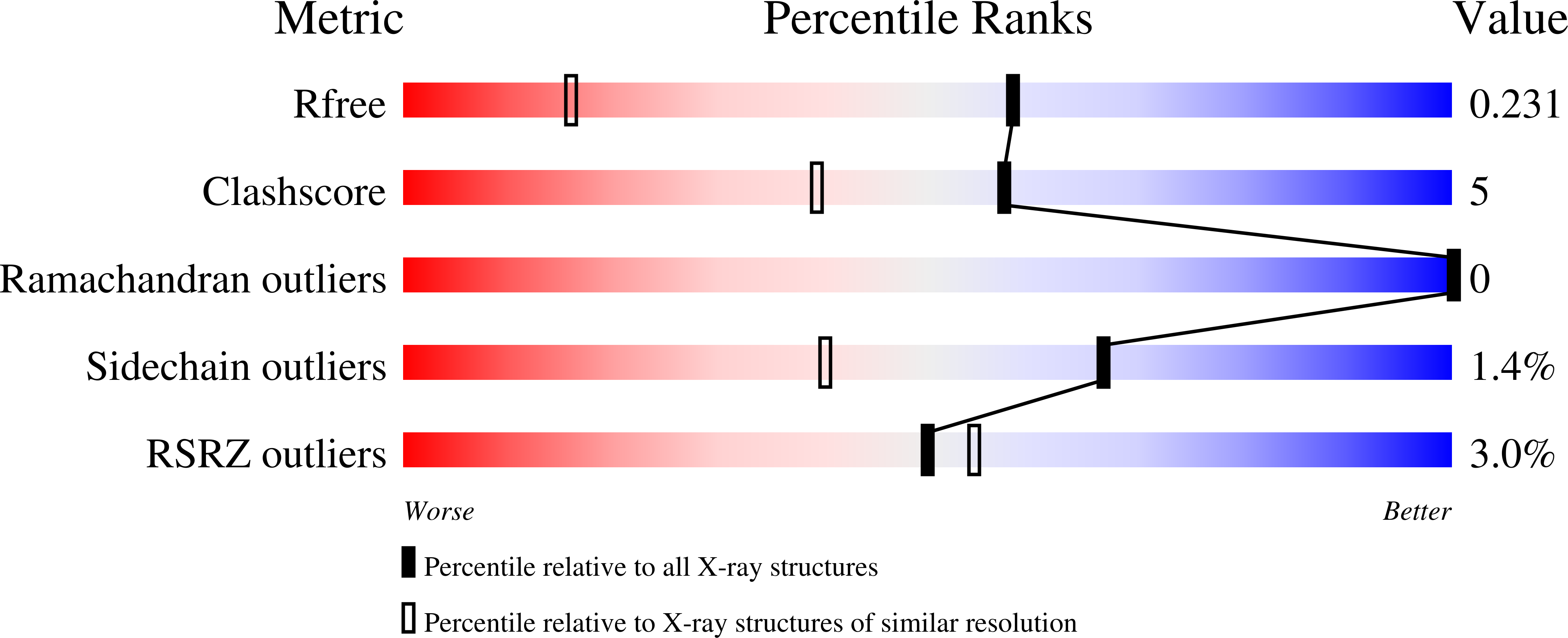
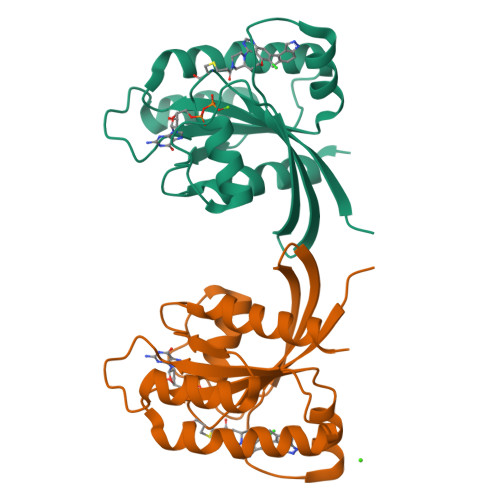
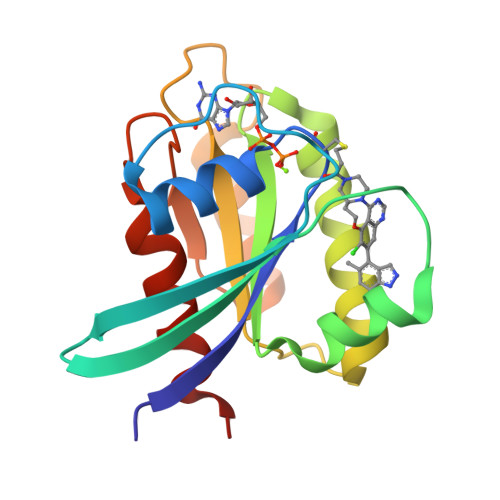
7O70, 7O83, 7OO7 - PubMed Abstract:
KRAS is an archetypal high-value intractable oncology drug target. The glycine to cysteine mutation at codon 12 represents an Achilles heel that has now rendered this important GTPase druggable. Herein, we report our structure-based drug design approach that led to the identification of 21 , AZD4625, a clinical development candidate for the treatment of KRAS G12C positive tumors. Highlights include a quinazoline tethering strategy to lock out a bio-relevant binding conformation and an optimization strategy focused on the reduction of extrahepatic clearance mechanisms seen in preclinical species. Crystallographic analysis was also key in helping to rationalize unusual structure-activity relationship in terms of ring size and enantio-preference. AZD4625 is a highly potent and selective inhibitor of KRAS G12C with an anticipated low clearance and high oral bioavailability profile in humans.
Organizational Affiliation:
Oncology R&D, AstraZeneca, Cambridge CB4 0WG, U.K.