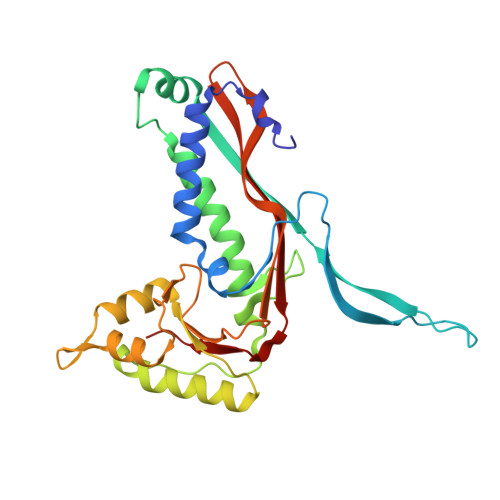
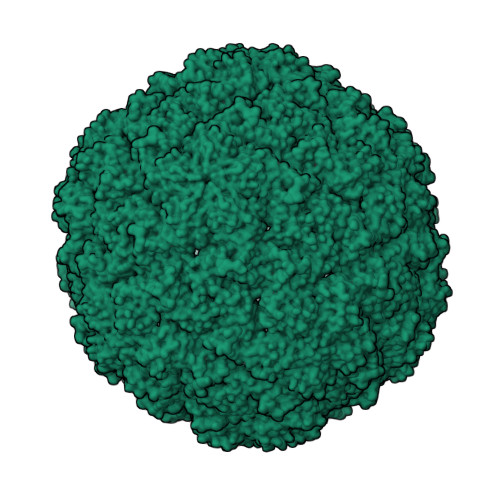
Pore dynamics and asymmetric cargo loading in an encapsulin nanocompartment.
Ross, J., McIver, Z., Lambert, T., Piergentili, C., Bird, J.E., Gallagher, K.J., Cruickshank, F.L., James, P., Zarazua-Arvizu, E., Horsfall, L.E., Waldron, K.J., Wilson, M.D., Mackay, C.L., Basle, A., Clarke, D.J., Marles-Wright, J.(2022) Sci Adv 8: eabj4461-eabj4461
- PubMed: 35080974
- DOI: https://doi.org/10.1126/sciadv.abj4461
- Primary Citation of Related Structures:
7ODW, 7OE2, 7OEU - PubMed Abstract:
Encapsulins are protein nanocompartments that house various cargo enzymes, including a family of decameric ferritin-like proteins. Here, we study a recombinant Haliangium ochraceum encapsulin:encapsulated ferritin complex using cryo-electron microscopy and hydrogen/deuterium exchange mass spectrometry to gain insight into the structural relationship between the encapsulin shell and its protein cargo. An asymmetric single-particle reconstruction reveals four encapsulated ferritin decamers in a tetrahedral arrangement within the encapsulin nanocompartment. This leads to a symmetry mismatch between the protein cargo and the icosahedral encapsulin shell. The encapsulated ferritin decamers are offset from the interior face of the encapsulin shell. Using hydrogen/deuterium exchange mass spectrometry, we observed the dynamic behavior of the major fivefold pore in the encapsulin shell and show the pore opening via the movement of the encapsulin A-domain. These data will accelerate efforts to engineer the encapsulation of heterologous cargo proteins and to alter the permeability of the encapsulin shell via pore modifications.
Organizational Affiliation:
EaStCHEM School of Chemistry, University of Edinburgh, Joseph Black Building, David Brewster Road, Edinburgh EH9 3FJ, UK.