Iron Binding in the Ferroxidase Site of Human Mitochondrial Ferritin.
Ciambellotti, S., Pratesi, A., Tassone, G., Turano, P., Mangani, S., Pozzi, C.(2021) Chemistry 27: 14690-14701
- PubMed: 34343376
- DOI: https://doi.org/10.1002/chem.202102270
- Primary Citation of Related Structures:
7O63, 7O64, 7O65, 7O66, 7O67, 7O68, 7O69, 7O6A, 7O6C, 7O6D, 7OWY - PubMed Abstract:
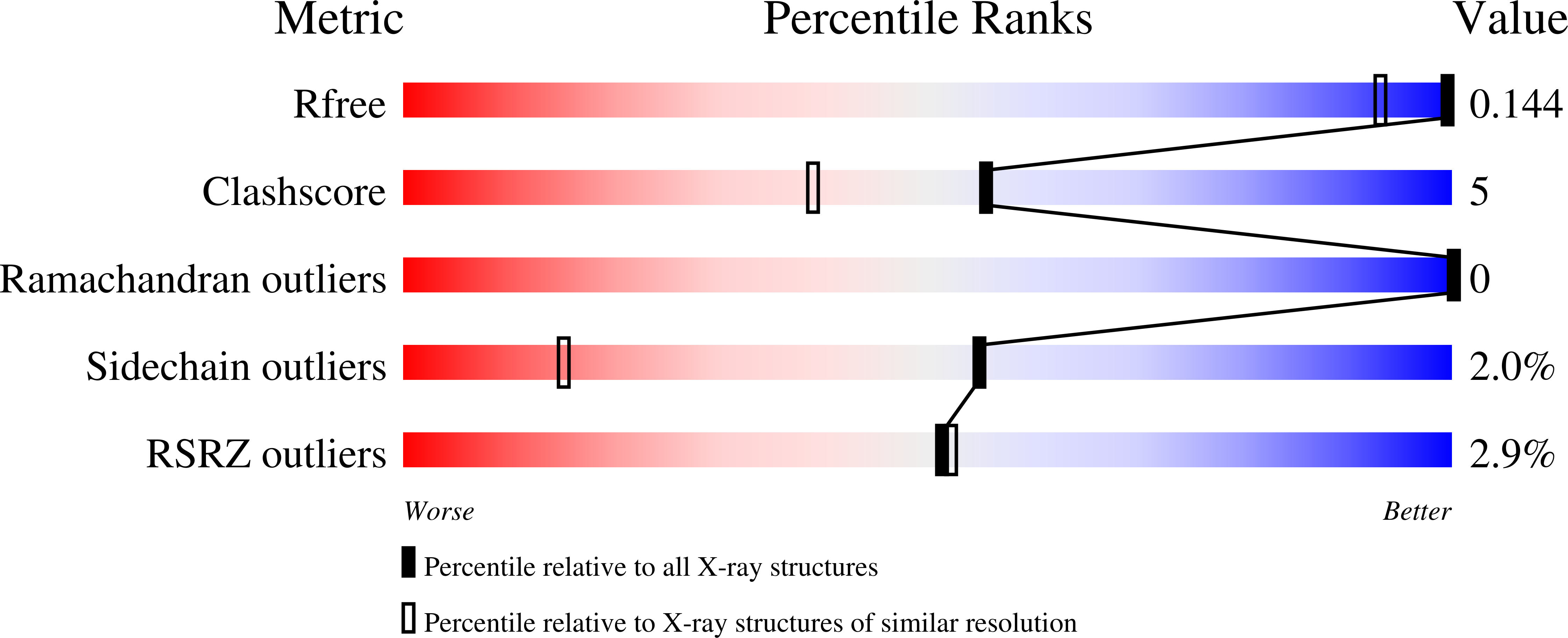
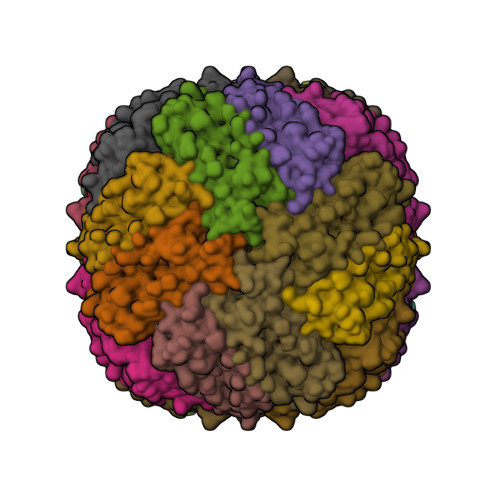
Ferritins are nanocage proteins that store iron ions in their central cavity as hydrated ferric oxide biominerals. In mammals, further the L (light) and H (heavy) chains constituting cytoplasmic maxi-ferritins, an additional type of ferritin has been identified, the mitochondrial ferritin (MTF). Human MTF (hMTF) is a functional homopolymeric H-like ferritin performing the ferroxidase activity in its ferroxidase site (FS), in which Fe(II) is oxidized to Fe(III) in the presence of dioxygen. To better investigate its ferroxidase properties, here we performed time-lapse X-ray crystallography analysis of hMTF, providing structural evidence of how iron ions interact with hMTF and of their binding to the FS. Transient iron binding sites, populating the pathway along the cage from the iron entry channel to the catalytic center, were also identified. Furthermore, our kinetic data at variable iron loads indicate that the catalytic iron oxidation reaction occurs via a diferric peroxo intermediate followed by the formation of ferric-oxo species, with significant differences with respect to human H-type ferritin.
Organizational Affiliation:
Department of Chemistry "Ugo Schiff" Department of Excellence 2018-2022, University of Florence, via della Lastruccia 2, 50019, Sesto Fiorentino, Italy.