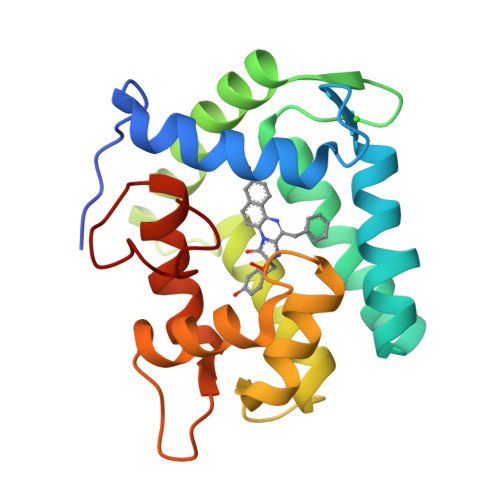
Crystal structure of semisynthetic obelin-v.
Larionova, M.D., Wu, L., Eremeeva, E.V., Natashin, P.V., Gulnov, D.V., Nemtseva, E.V., Liu, D., Liu, Z.J., Vysotski, E.S.(2022) Protein Sci 31: 454-469
- PubMed: 34802167
- DOI: https://doi.org/10.1002/pro.4244
- Primary Citation of Related Structures:
7O3U - PubMed Abstract:
Coelenterazine-v (CTZ-v), a synthetic derivative with an additional benzyl ring, yields a bright bioluminescence of Renilla luciferase and its "yellow" mutant with a significant shift in the emission spectrum toward longer wavelengths, which makes it the substrate of choice for deep tissue imaging. Although Ca 2+ -regulated photoproteins activated with CTZ-v also display red-shifted light emission, in contrast to Renilla luciferase their bioluminescence activities are very low, which makes photoproteins activated by CTZ-v unusable for calcium imaging. Here, we report the crystal structure of Ca 2+ -regulated photoprotein obelin with 2-hydroperoxycoelenterazine-v (obelin-v) at 1.80 Å resolution. The structures of obelin-v and obelin bound with native CTZ revealed almost no difference; only the minor rearrangement in hydrogen-bond pattern and slightly increased distances between key active site residues and some atoms of 2-hydroperoxycoelenterazine-v were found. The fluorescence quantum yield (Φ FL ) of obelin bound with coelenteramide-v (0.24) turned out to be even higher than that of obelin with native coelenteramide (0.19). Since both obelins are in effect the enzyme-substrate complexes containing the 2-hydroperoxy adduct of CTZ-v or CTZ, we reasonably assume the chemical reaction mechanisms and the yields of the reaction products (Φ R ) to be similar for both obelins. Based on these findings we suggest that low bioluminescence activity of obelin-v is caused by the low efficiency of generating an electronic excited state (Φ S ). In turn, the low Φ S value as compared to that of native CTZ might be the result of small changes in the substrate microenvironment in the obelin-v active site.
Organizational Affiliation:
Photobiology Laboratory, Institute of Biophysics SB RAS, Federal Research Center "Krasnoyarsk Science Center SB RAS", Krasnoyarsk, Russia.