Dynamic acylome reveals metabolite driven modifications in Syntrophomonas wolfei.
Fu, J.Y., Muroski, J.M., Arbing, M.A., Salguero, J.A., Wofford, N.Q., McInerney, M.J., Gunsalus, R.P., Loo, J.A., Ogorzalek Loo, R.R.(2022) Front Microbiol 13: 1018220-1018220
- PubMed: 36419437
- DOI: https://doi.org/10.3389/fmicb.2022.1018220
- Primary Citation of Related Structures:
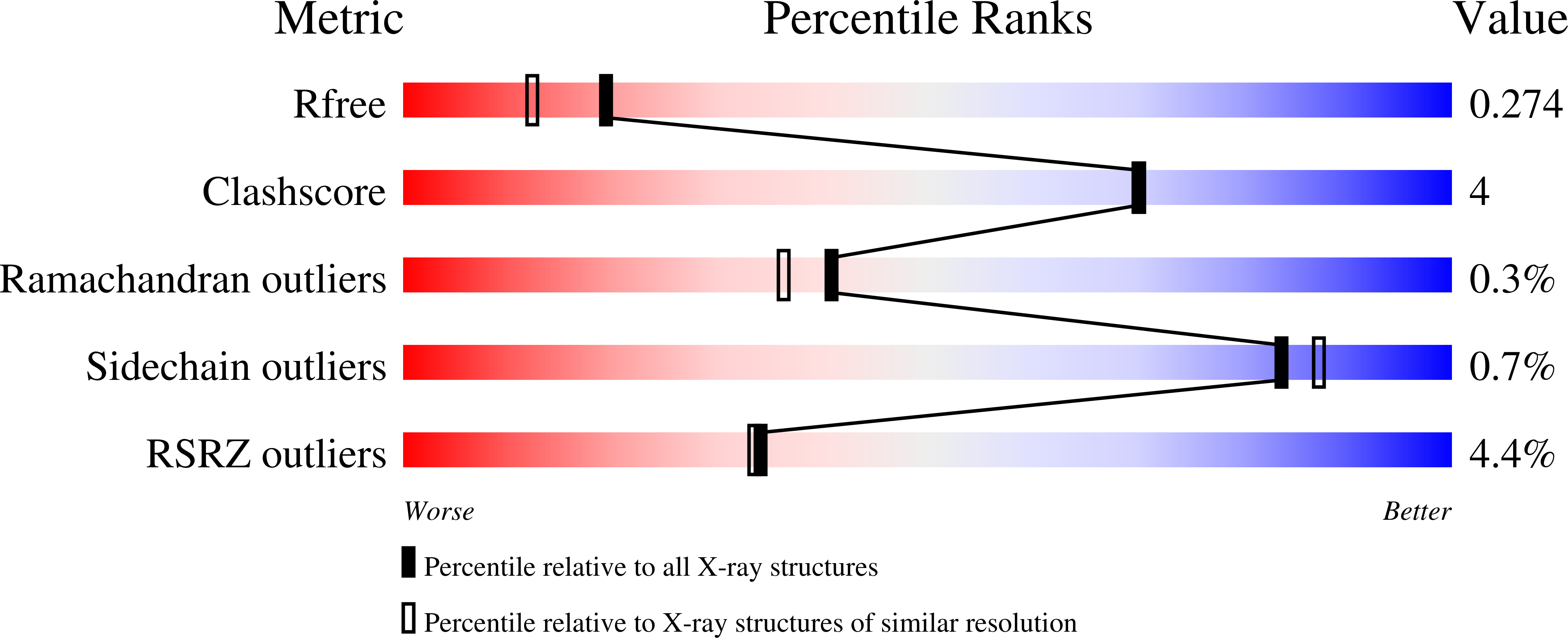
7N7Z - PubMed Abstract:
Syntrophomonas wolfei is an anaerobic syntrophic microbe that degrades short-chain fatty acids to acetate, hydrogen, and/or formate. This thermodynamically unfavorable process proceeds through a series of reactive acyl-Coenzyme A species (RACS). In other prokaryotic and eukaryotic systems, the production of intrinsically reactive metabolites correlates with acyl-lysine modifications, which have been shown to play a significant role in metabolic processes. Analogous studies with syntrophic bacteria, however, are relatively unexplored and we hypothesized that highly abundant acylations could exist in S. wolfei proteins, corresponding to the RACS derived from degrading fatty acids. Here, by mass spectrometry-based proteomics (LC-MS/MS), we characterize and compare acylome profiles of two S. wolfei subspecies grown on different carbon substrates. Because modified S. wolfei proteins are sufficiently abundant to analyze post-translational modifications (PTMs) without antibody enrichment, we could identify types of acylations comprehensively, observing six types (acetyl-, butyryl-, 3- hydroxybutyryl-, crotonyl-, valeryl-, and hexanyl-lysine), two of which have not been reported in any system previously. All of the acyl-PTMs identified correspond directly to RACS in fatty acid degradation pathways. A total of 369 sites of modification were identified on 237 proteins. Structural studies and in vitro acylation assays of a heavily modified enzyme, acetyl-CoA transferase, provided insight on the potential impact of these acyl-protein modifications. The extensive changes in acylation-type, abundance, and modification sites with carbon substrate suggest that protein acylation by RACS may be an important regulator of syntrophy.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA, United States.