Structural basis of omega-3 fatty acid transport across the blood-brain barrier.
Cater, R.J., Chua, G.L., Erramilli, S.K., Keener, J.E., Choy, B.C., Tokarz, P., Chin, C.F., Quek, D.Q.Y., Kloss, B., Pepe, J.G., Parisi, G., Wong, B.H., Clarke, O.B., Marty, M.T., Kossiakoff, A.A., Khelashvili, G., Silver, D.L., Mancia, F.(2021) Nature 595: 315-319
- PubMed: 34135507
- DOI: https://doi.org/10.1038/s41586-021-03650-9
- Primary Citation of Related Structures:
7MJS - PubMed Abstract:
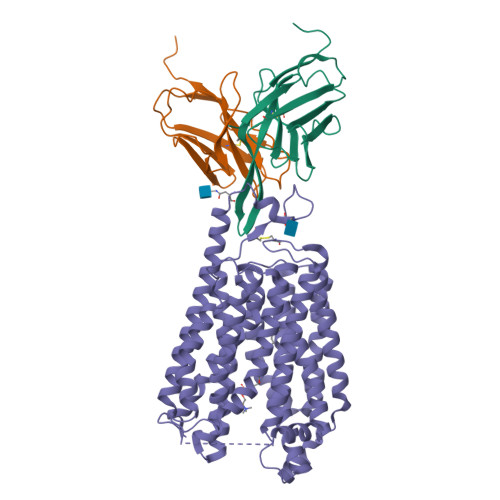
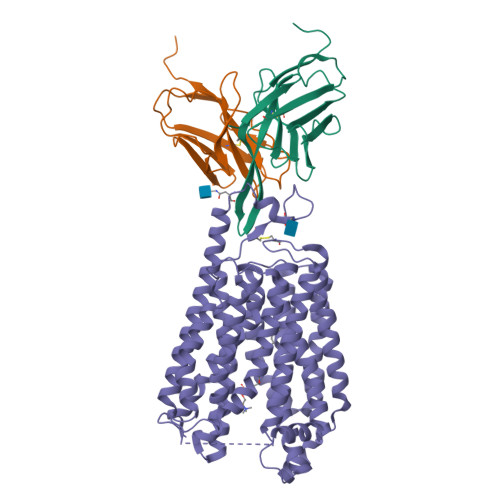
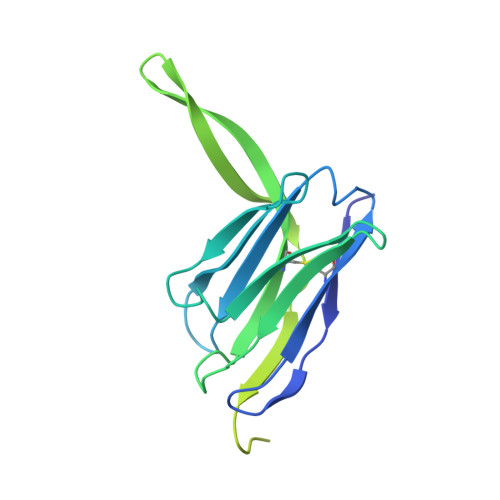
Docosahexaenoic acid is an omega-3 fatty acid that is essential for neurological development and function, and it is supplied to the brain and eyes predominantly from dietary sources 1-6 . This nutrient is transported across the blood-brain and blood-retina barriers in the form of lysophosphatidylcholine by major facilitator superfamily domain containing 2A (MFSD2A) in a Na + -dependent manner 7,8 . Here we present the structure of MFSD2A determined using single-particle cryo-electron microscopy, which reveals twelve transmembrane helices that are separated into two pseudosymmetric domains. The transporter is in an inward-facing conformation and features a large amphipathic cavity that contains the Na + -binding site and a bound lysolipid substrate, which we confirmed using native mass spectrometry. Together with our functional analyses and molecular dynamics simulations, this structure reveals details of how MFSD2A interacts with substrates and how Na + -dependent conformational changes allow for the release of these substrates into the membrane through a lateral gate. Our work provides insights into the molecular mechanism by which this atypical major facility superfamily transporter mediates the uptake of lysolipids into the brain, and has the potential to aid in the delivery of neurotherapeutic agents.
Organizational Affiliation:
Department of Physiology and Cellular Biophysics, Columbia University, New York, NY, USA.