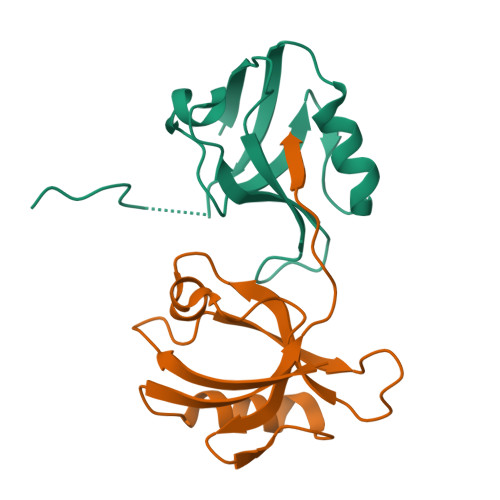
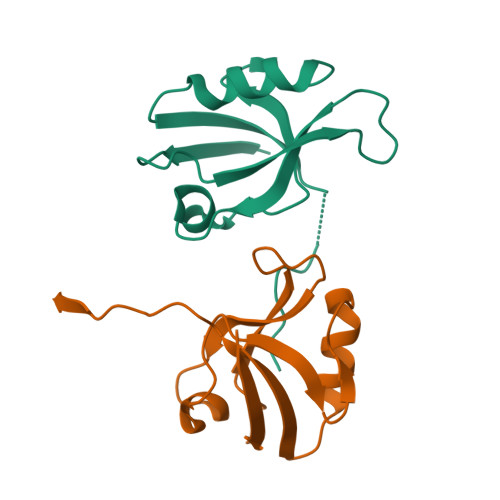
Multivalent interactions make adherens junction-cytoskeletal linkage robust during morphogenesis.
Perez-Vale, K.Z., Yow, K.D., Johnson, R.I., Byrnes, A.E., Finegan, T.M., Slep, K.C., Peifer, M.(2021) J Cell Biol 220
- PubMed: 34762121
- DOI: https://doi.org/10.1083/jcb.202104087
- Primary Citation of Related Structures:
7MFW - PubMed Abstract:
Embryogenesis requires cells to change shape and move without disrupting epithelial integrity. This requires robust, responsive linkage between adherens junctions and the actomyosin cytoskeleton. Using Drosophila morphogenesis, we define molecular mechanisms mediating junction-cytoskeletal linkage and explore the role of mechanosensing. We focus on the junction-cytoskeletal linker Canoe, a multidomain protein. We engineered the canoe locus to define how its domains mediate its mechanism of action. To our surprise, the PDZ and FAB domains, which we thought connected junctions and F-actin, are not required for viability or mechanosensitive recruitment to junctions under tension. The FAB domain stabilizes junctions experiencing elevated force, but in its absence, most cells recover, suggesting redundant interactions. In contrast, the Rap1-binding RA domains are critical for all Cno functions and enrichment at junctions under tension. This supports a model in which junctional robustness derives from a large protein network assembled via multivalent interactions, with proteins at network nodes and some node connections more critical than others.
Organizational Affiliation:
Curriculum in Genetics and Molecular Biology, University of North Carolina at Chapel Hill, Chapel Hill, NC.