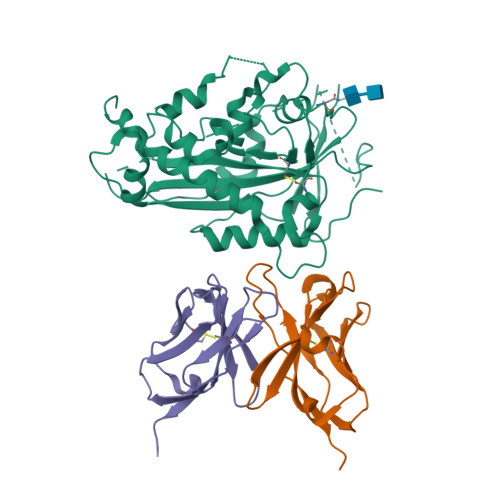
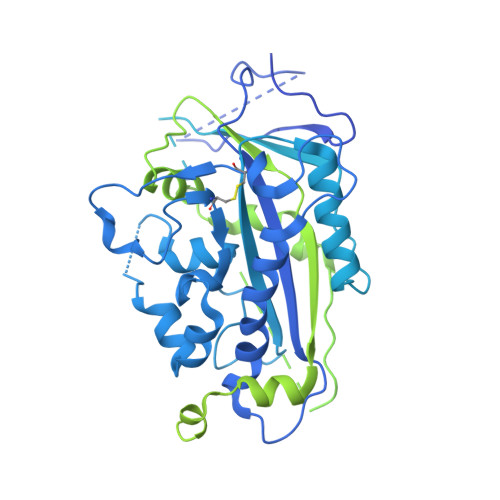
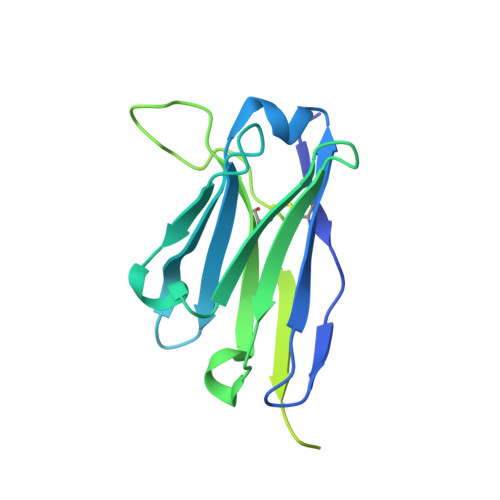
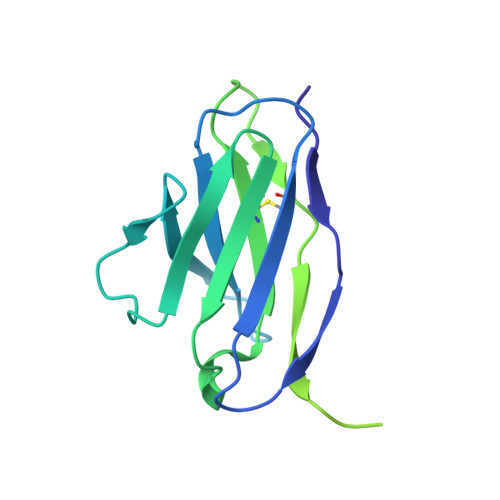
Scap structures highlight key role for rotation of intertwined luminal loops in cholesterol sensing.
Kober, D.L., Radhakrishnan, A., Goldstein, J.L., Brown, M.S., Clark, L.D., Bai, X.C., Rosenbaum, D.M.(2021) Cell 184: 3689
- PubMed: 34139175
- DOI: https://doi.org/10.1016/j.cell.2021.05.019
- Primary Citation of Related Structures:
7LKF, 7LKH - PubMed Abstract:
The cholesterol-sensing protein Scap induces cholesterol synthesis by transporting membrane-bound transcription factors called sterol regulatory element-binding proteins (SREBPs) from the endoplasmic reticulum (ER) to the Golgi apparatus for proteolytic activation. Transport requires interaction between Scap's two ER luminal loops (L1 and L7), which flank an intramembrane sterol-sensing domain (SSD). Cholesterol inhibits Scap transport by binding to L1, which triggers Scap's binding to Insig, an ER retention protein. Here we used cryoelectron microscopy (cryo-EM) to elucidate two structures of full-length chicken Scap: (1) a wild-type free of Insigs and (2) mutant Scap bound to chicken Insig without cholesterol. Strikingly, L1 and L7 intertwine tightly to form a globular domain that acts as a luminal platform connecting the SSD to the rest of Scap. In the presence of Insig, this platform undergoes a large rotation accompanied by rearrangement of Scap's transmembrane helices. We postulate that this conformational change halts Scap transport of SREBPs and inhibits cholesterol synthesis.
Organizational Affiliation:
Department of Biophysics, The University of Texas Southwestern Medical Center, Dallas, TX 75390, USA; Department of Molecular Genetics, The University of Texas Southwestern Medical Center, Dallas, TX 75390, USA.