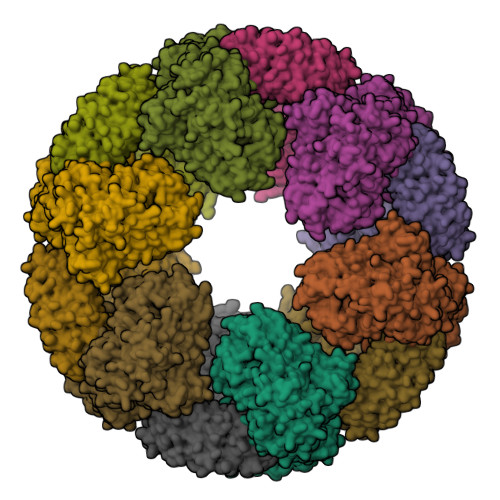
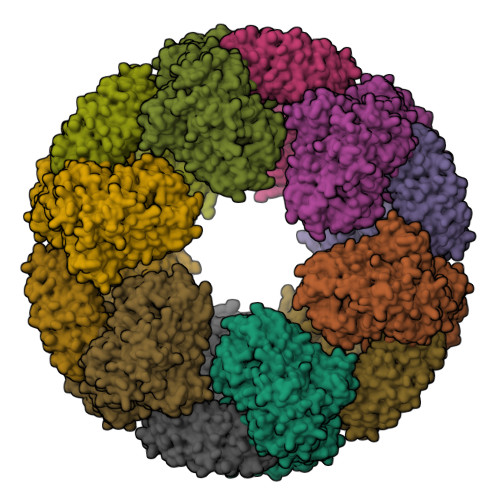
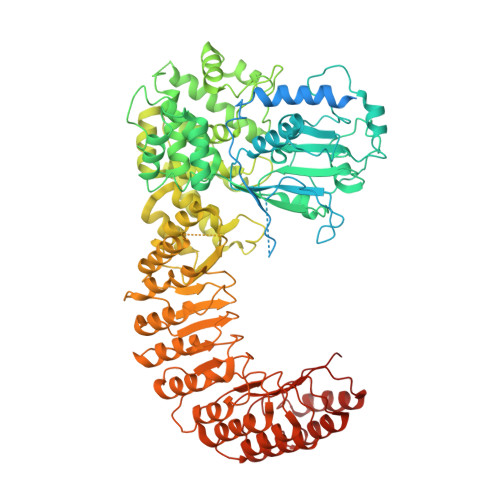
NLRP3 cages revealed by full-length mouse NLRP3 structure control pathway activation.
Andreeva, L., David, L., Rawson, S., Shen, C., Pasricha, T., Pelegrin, P., Wu, H.(2021) Cell 184: 6299
- PubMed: 34861190
- DOI: https://doi.org/10.1016/j.cell.2021.11.011
- Primary Citation of Related Structures:
7LFH - PubMed Abstract:
The NACHT-, leucine-rich-repeat- (LRR), and pyrin domain-containing protein 3 (NLRP3) is emerging to be a critical intracellular inflammasome sensor of membrane integrity and a highly important clinical target against chronic inflammation. Here, we report that an endogenous, stimulus-responsive form of full-length mouse NLRP3 is a 12- to 16-mer double-ring cage held together by LRR-LRR interactions with the pyrin domains shielded within the assembly to avoid premature activation. Surprisingly, this NLRP3 form is predominantly membrane localized, which is consistent with previously noted localization of NLRP3 at various membrane organelles. Structure-guided mutagenesis reveals that trans-Golgi network dispersion into vesicles, an early event observed for many NLRP3-activating stimuli, requires the double-ring cages of NLRP3. Double-ring-defective NLRP3 mutants abolish inflammasome punctum formation, caspase-1 processing, and cell death. Thus, our data uncover a physiological NLRP3 oligomer on the membrane that is poised to sense diverse signals to induce inflammasome activation.
Organizational Affiliation:
Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA; Program in Cellular and Molecular Medicine, Boston Children's Hospital, Boston, MA 02115, USA.