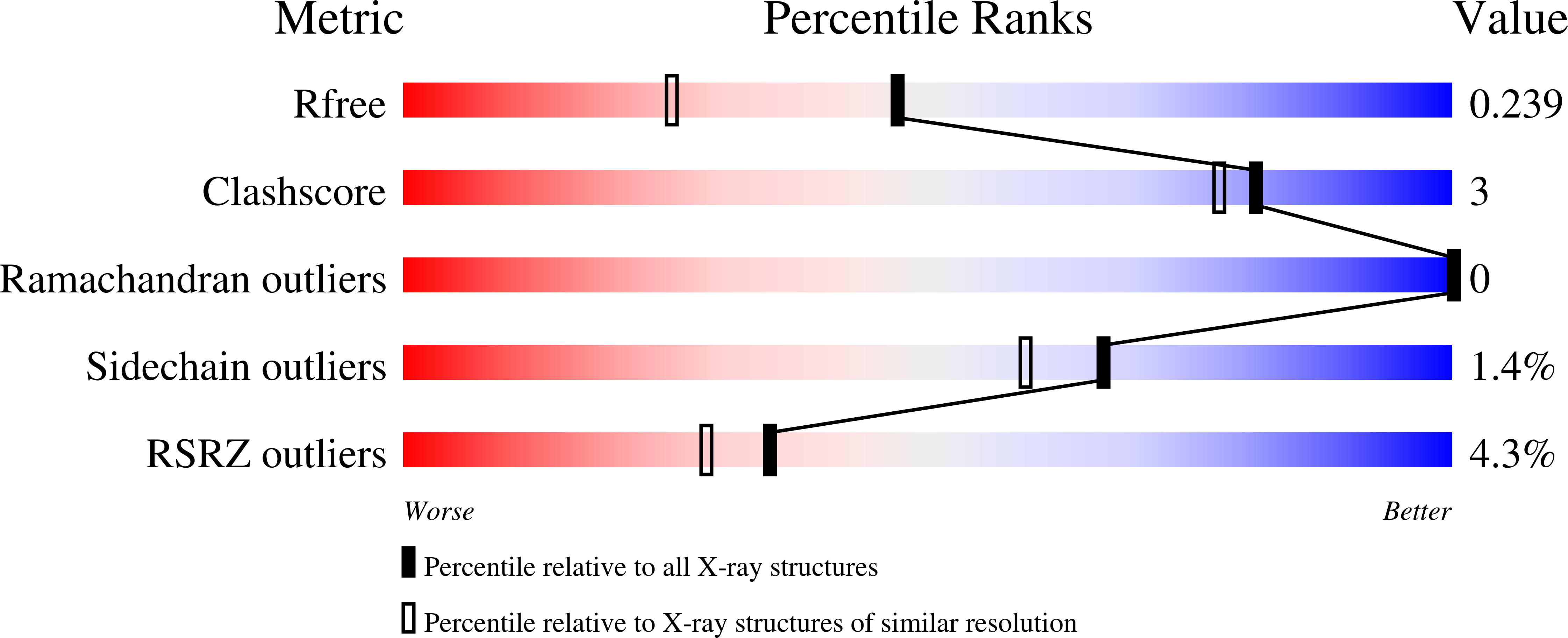
Human MettL3-MettL14 RNA adenine methyltransferase complex is active on double-stranded DNA containing lesions.
Yu, D., Horton, J.R., Yang, J., Hajian, T., Vedadi, M., Sagum, C.A., Bedford, M.T., Blumenthal, R.M., Zhang, X., Cheng, X.(2021) Nucleic Acids Res 49: 11629-11642
- PubMed: 34086966
- DOI: https://doi.org/10.1093/nar/gkab460
- Primary Citation of Related Structures:
7L4X, 7L4Y - PubMed Abstract:
MettL3-MettL14 methyltransferase complex has been studied widely for its role in RNA adenine methylation. This complex is also recruited to UV- and X-ray exposed DNA damaged sites, and its methyltransfer activity is required for subsequent DNA repair, though in theory this could result from RNA methylation of short transcripts made at the site of damage. We report here that MettL3-MettL14 is active in vitro on double-stranded DNA containing a cyclopyrimidine dimer - a major lesion of UV radiation-induced products - or an abasic site or mismatches. Furthermore, N6-methyladenine (N6mA) decreases misincorporation of 8-oxo-guanine (8-oxoG) opposite to N6mA by repair DNA polymerases. When 8-oxoG is nevertheless incorporated opposite N6mA, the methylation inhibits N6mA excision from the template (correct) strand by the adenine DNA glycosylase (MYH), implying that the methylation decreases inappropriate misrepair. Finally, we observed that the N6mA reader domain of YTHDC1, which is also recruited to sites of DNA damage, binds N6mA that is located across from a single-base gap between two canonical DNA helices. This YTHDC1 complex with a gapped duplex is structurally similar to DNA complexes with FEN1 and GEN1 - two members of the nuclease family that act in nucleotide excision repair, mismatch repair and homologous recombination, and which incise distinct non-B DNA structures. Together, the parts of our study provide a plausible mechanism for N6mA writer and reader proteins acting directly on lesion-containing DNA, and suggest in vivo experiments to test the mechanisms involving methylation of adenine.
Organizational Affiliation:
Department of Epigenetics and Molecular Carcinogenesis, University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.