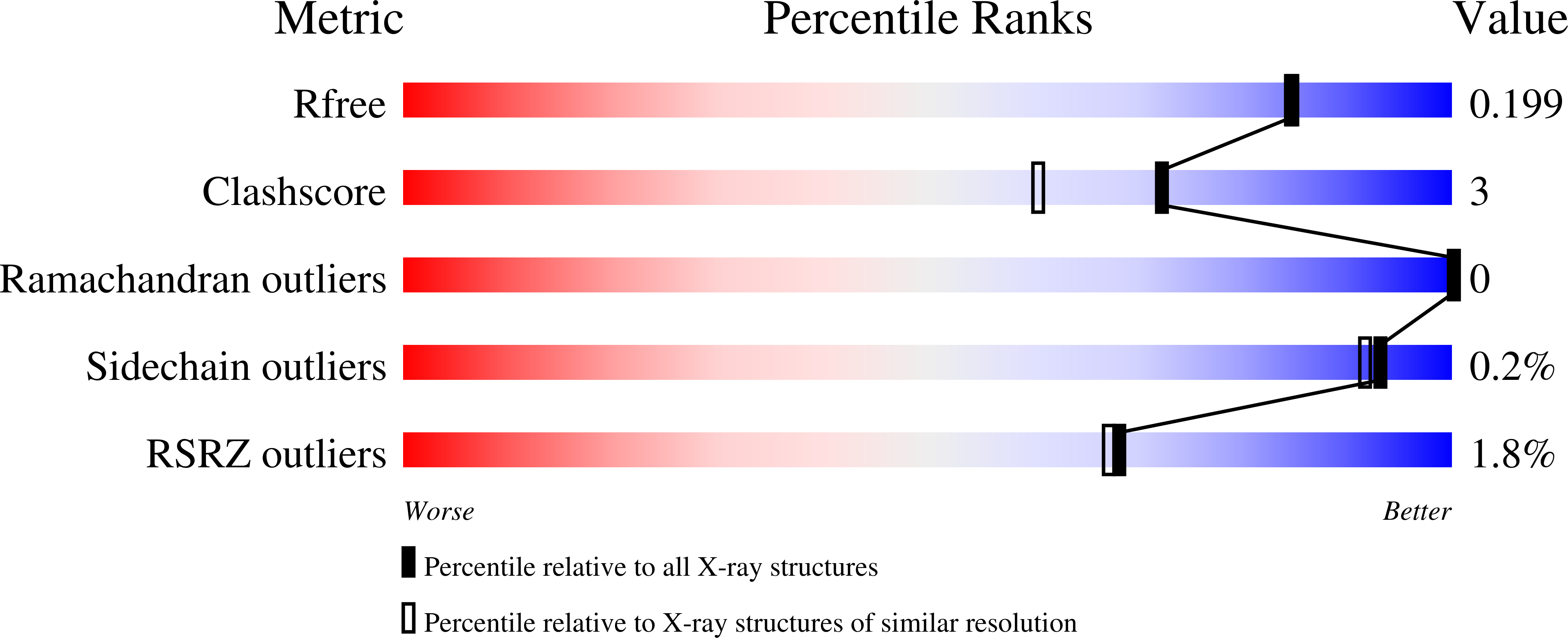
Millisecond mix-and-quench crystallography (MMQX) enables time-resolved studies of PEPCK with remote data collection.
Clinger, J.A., Moreau, D.W., McLeod, M.J., Holyoak, T., Thorne, R.E.(2021) IUCrJ 8: 784-792
- PubMed: 34584739
- DOI: https://doi.org/10.1107/S2052252521007053
- Primary Citation of Related Structures:
7L36, 7L3M, 7L3V - PubMed Abstract:
Time-resolved crystallography of biomolecules in action has advanced rapidly as methods for serial crystallography have improved, but the large number of crystals and the complex experimental infrastructure that are required remain serious obstacles to its widespread application. Here, millisecond mix-and-quench crystallography (MMQX) has been developed, which yields millisecond time-resolved data using far fewer crystals and routine remote synchrotron data collection. To demonstrate the capabilities of MMQX, the conversion of oxaloacetic acid to phosphoenolpyruvate by phosphoenolpyruvate carboxy-kinase (PEPCK) is observed with a time resolution of 40 ms. By lowering the entry barrier to time-resolved crystallography, MMQX should enable a broad expansion in structural studies of protein dynamics.
Organizational Affiliation:
Physics Department, Cornell University, 142 Sciences Drive, Ithaca, NY 14853, USA.