Structural Analysis of the Macrocyclic Inhibitor BI-4020 Binding to EGFR Kinase.
Beyett, T.S., Rana, J.K., Schaeffner, I.K., Heppner, D.E., Eck, M.J.(2024) ChemMedChem : e202300343-e202300343
- PubMed: 38523074
- DOI: https://doi.org/10.1002/cmdc.202300343
- Primary Citation of Related Structures:
7KXZ, 7KY0 - PubMed Abstract:
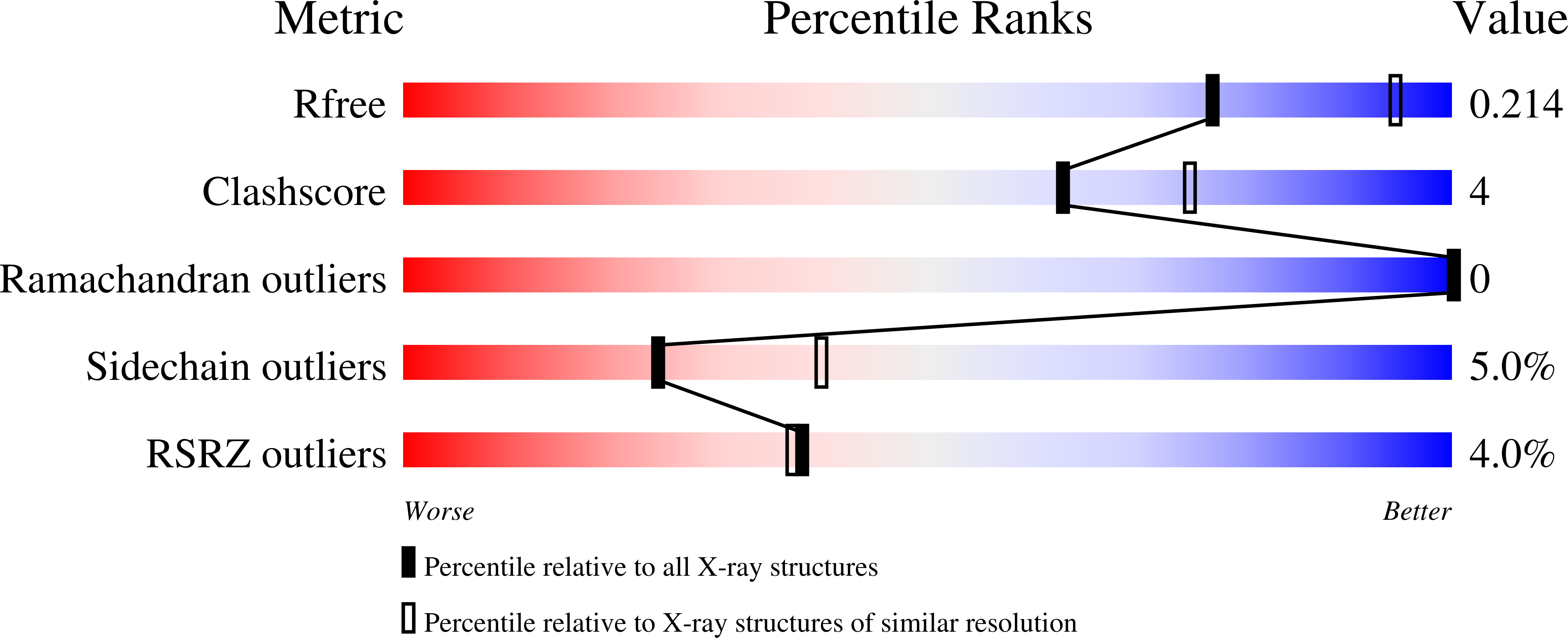
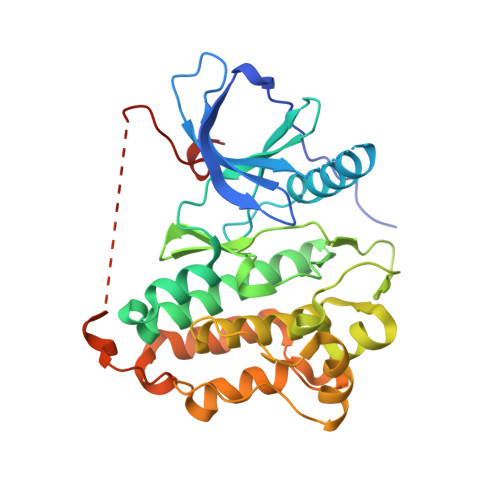
A novel macrocyclic inhibitor of mutant EGFR (BI-4020) has shown promise in pre-clinical studies of T790M and C797S drug-resistant non-small cell lung cancer. To better understand the molecular basis for BI-4020 selectivity and potency, we have carried out biochemical activity assays and structural analysis with X-ray crystallography. Biochemical potencies agree with previous studies indicating that BI-4020 is uniquely potent against drug-resistant L858R/T790M and L858R/T790M/C797S variants. X-ray structures with wild-type (2.4 Å) and T790M/V948R (3.1 Å) EGFR kinase domains show that BI-4020 is likely rendered selective due to interactions with the kinase domain hinge region as well as T790M, akin to Osimertinib. Additionally, BI-4020 is also rendered more potent due to its constrained macrocycle geometry as well as additional H-bonds to conserved K745 and T845 residues in both active and inactive conformations. These findings taken together show how this novel macrocyclic inhibitor is both highly potent and selective for mutant EGFR in a reversible mechanism and motivate structure-inspired approaches to developing targeted therapies in medicinal oncology.
Organizational Affiliation:
Department of Cancer Biology, Dana-Farber Cancer Institute, Boston, MA 02215, USA, 450 Brookline Avenue, LC4313.