Structure-Guided Development of Small-Molecule PRC2 Inhibitors Targeting EZH2-EED Interaction.
Du, D., Xu, D., Zhu, L., Stazi, G., Zwergel, C., Liu, Y., Luo, Z., Li, Y., Zhang, Y., Zhu, K., Ding, Y., Liu, J., Fan, S., Zhao, K., Zhang, N., Kong, X., Jiang, H., Chen, K., Zhao, K., Valente, S., Min, J., Duan, W., Luo, C.(2021) J Med Chem 64: 8194-8207
- PubMed: 34077206
- DOI: https://doi.org/10.1021/acs.jmedchem.0c02261
- Primary Citation of Related Structures:
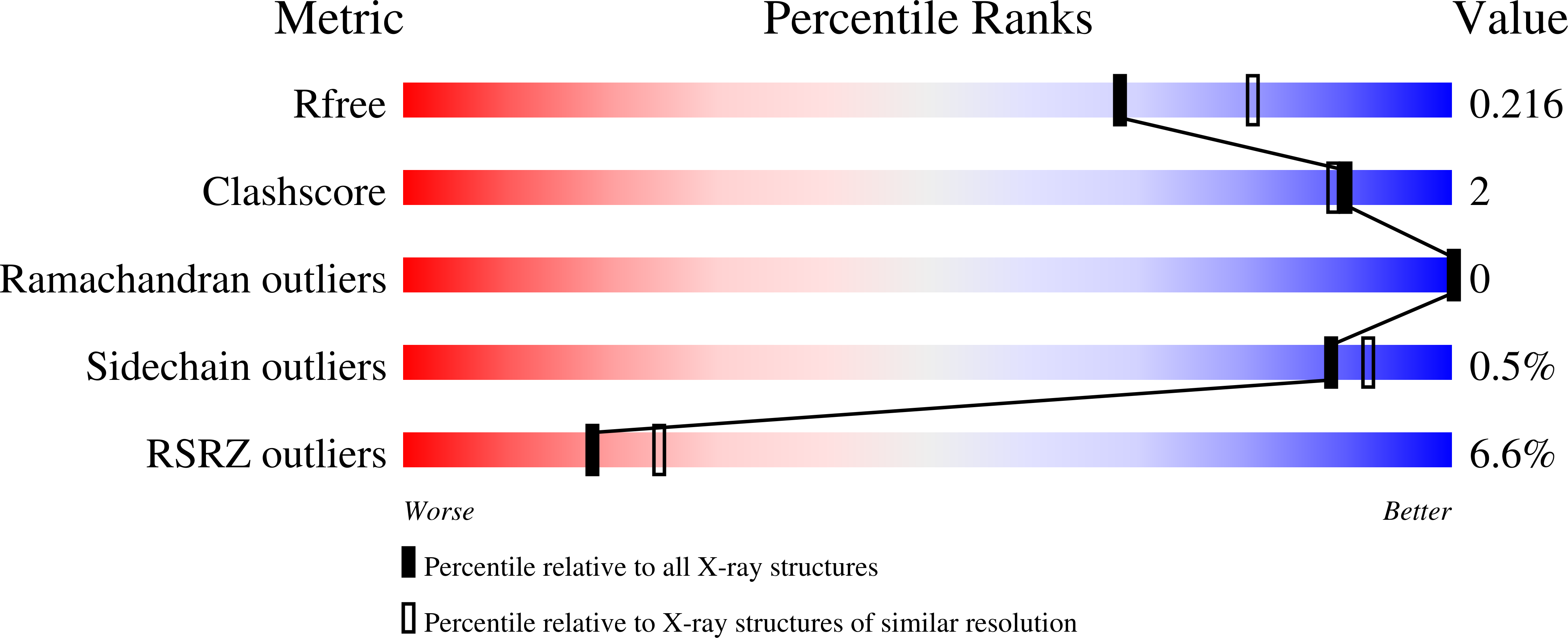
7KXT - PubMed Abstract:
Disruption of EZH2-embryonic ectoderm development (EED) protein-protein interaction (PPI) is a new promising cancer therapeutic strategy. We have previously reported the discovery of astemizole, a small-molecule inhibitor targeting the EZH2-EED PPI. Herein, we report the cocrystal structure of EED in complex with astemizole at 2.15 Å. The structure elucidates the detailed binding mode of astemizole to EED and provides a structure-guided design for the discovery of a novel EZH2-EED interaction inhibitor, DC-PRC2in-01, with an affinity K d of 4.56 μM. DC-PRC2in-01 destabilizes the PRC2 complex, thereby leading to the degradation of PRC2 core proteins and the decrease of global H3K27me3 levels in cancer cells. The proliferation of PRC2-driven lymphomas cells is effectively inhibited, and the cell cycle is arrested in the G0/G1 phase. Together, these data demonstrate that DC-PRC2in-01 could be an effective chemical probe for investigating the PRC2-related physiology and pathology and providing a promising chemical scaffold for further development.
Organizational Affiliation:
School of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing 210023 Jiangsu, China.