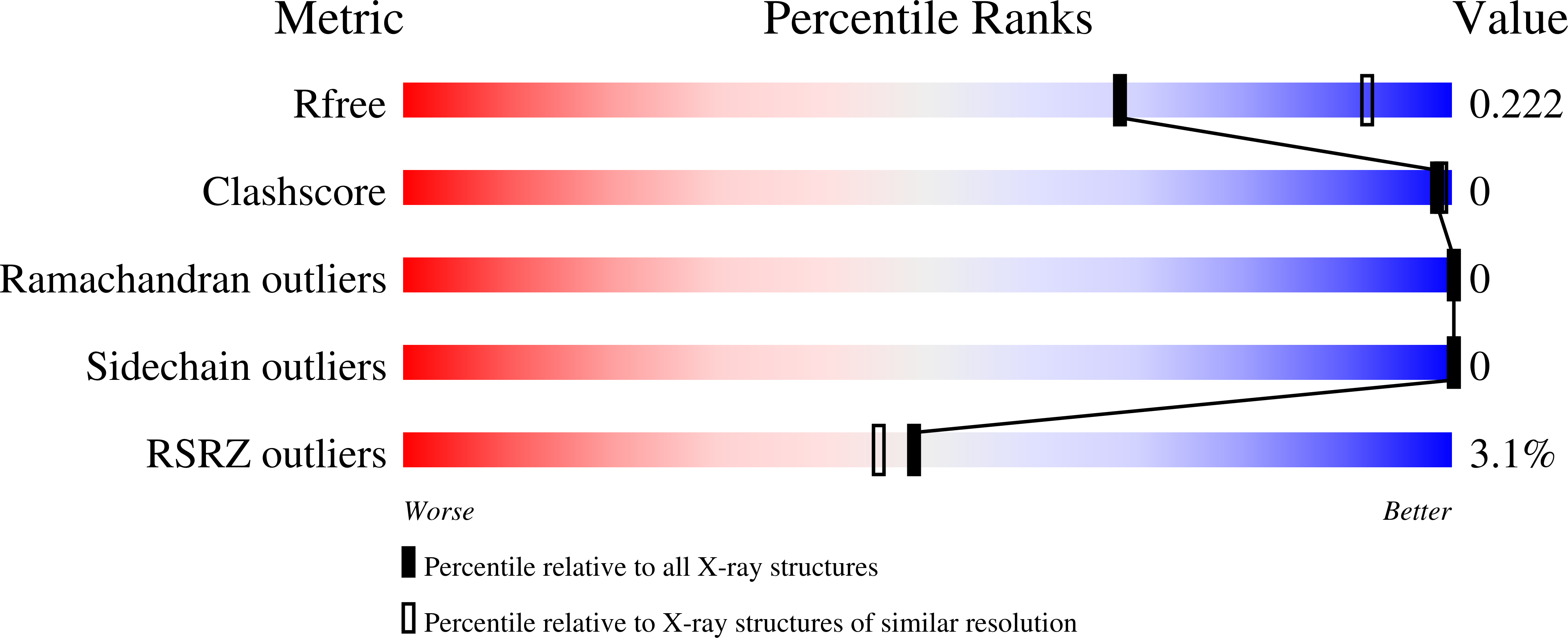
Discovery of Dual TAF1-ATR Inhibitors and Ligand-Induced Structural Changes of the TAF1 Tandem Bromodomain.
Karim, R.M., Yang, L., Chen, L., Bikowitz, M.J., Lu, J., Grassie, D., Shultz, Z.P., Lopchuk, J.M., Chen, J., Schonbrunn, E.(2022) J Med Chem 65: 4182-4200
- PubMed: 35191694
- DOI: https://doi.org/10.1021/acs.jmedchem.1c01999
- Primary Citation of Related Structures:
7JJG, 7JJH, 7JSP, 7K03, 7K0D, 7K0U, 7K1P, 7K27, 7K3O, 7K42, 7K6F, 7L6X - PubMed Abstract:
Bromodomains regulate chromatin remodeling and gene transcription through recognition of acetylated lysines on histones and other proteins. Bromodomain-containing protein TAF1, a subunit of general transcription factor TFIID, initiates preinitiation complex formation and cellular transcription. TAF1 serves as a cofactor for certain oncogenic transcription factors and is implicated in regulating the p53 tumor suppressor. Therefore, TAF1 is a potential target to develop small molecule therapeutics for diseases arising from dysregulated transcription, such as cancer. Here, we report the ATR kinase inhibitor AZD6738 (Ceralasertib) and analogues thereof as bona fide inhibitors of TAF1. Crystallographic and small-angle X-ray scattering studies established that newly identified and previously reported inhibitors stabilize distinct structural states of the TAF1 tandem bromodomain through "open-closed" transitions and dimerization. Combined with functional studies on p53 signaling in cancer cell lines, the data provide new insights into the feasibility and challenges of TAF1 inhibitors as chemical probes and therapeutics.
Organizational Affiliation:
Drug Discovery Department, Moffitt Cancer Center, Tampa, Florida 33612, United States.