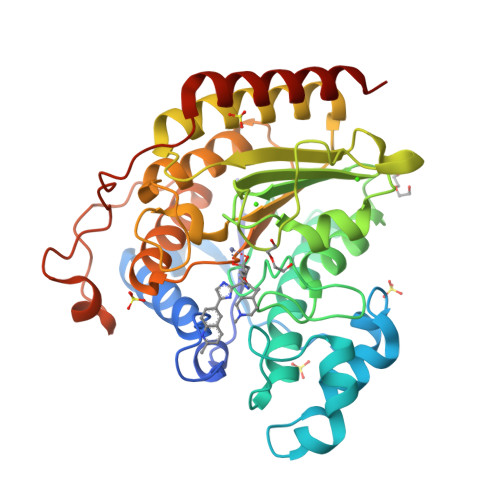
Discovery of Ethyl Ketone-Based Highly Selective HDACs 1, 2, 3 Inhibitors for HIV Latency Reactivation with Minimum Cellular Potency Serum Shift and Reduced hERG Activity.
Yu, W., Liu, J., Clausen, D., Yu, Y., Duffy, J.L., Wang, M., Xu, S., Deng, L., Suzuki, T., Chung, C.C., Myers, R.W., Klein, D.J., Fells, J.I., Holloway, M.K., Wu, J., Wu, G., Howell, B.J., Barnard, R.J.O., Kozlowski, J.(2021) J Med Chem 64: 4709-4729
- PubMed: 33797924
- DOI: https://doi.org/10.1021/acs.jmedchem.0c02150
- Primary Citation of Related Structures:
7JS8 - PubMed Abstract:
We describe the discovery of histone deacetylase (HDACs) 1, 2, and 3 inhibitors with ethyl ketone as the zinc-binding group. These HDACs 1, 2, and 3 inhibitors have good enzymatic and cellular activity. Their serum shift in cellular potency has been minimized, and selectivity against hERG has been improved. They are also highly selective over HDACs 6 and 8. These inhibitors contain a variety of substituted heterocycles on the imidazole or oxazole scaffold. Compounds 31 and 48 stand out due to their good potency, high selectivity over HDACs 6 and 8, reduced hERG activity, optimized serum shift in cellular potency, and good rat and dog PK profiles.
Organizational Affiliation:
Merck & Co. Inc., 2000 Galloping Hill Road, Kenilworth, New Jersey 07033, United States.