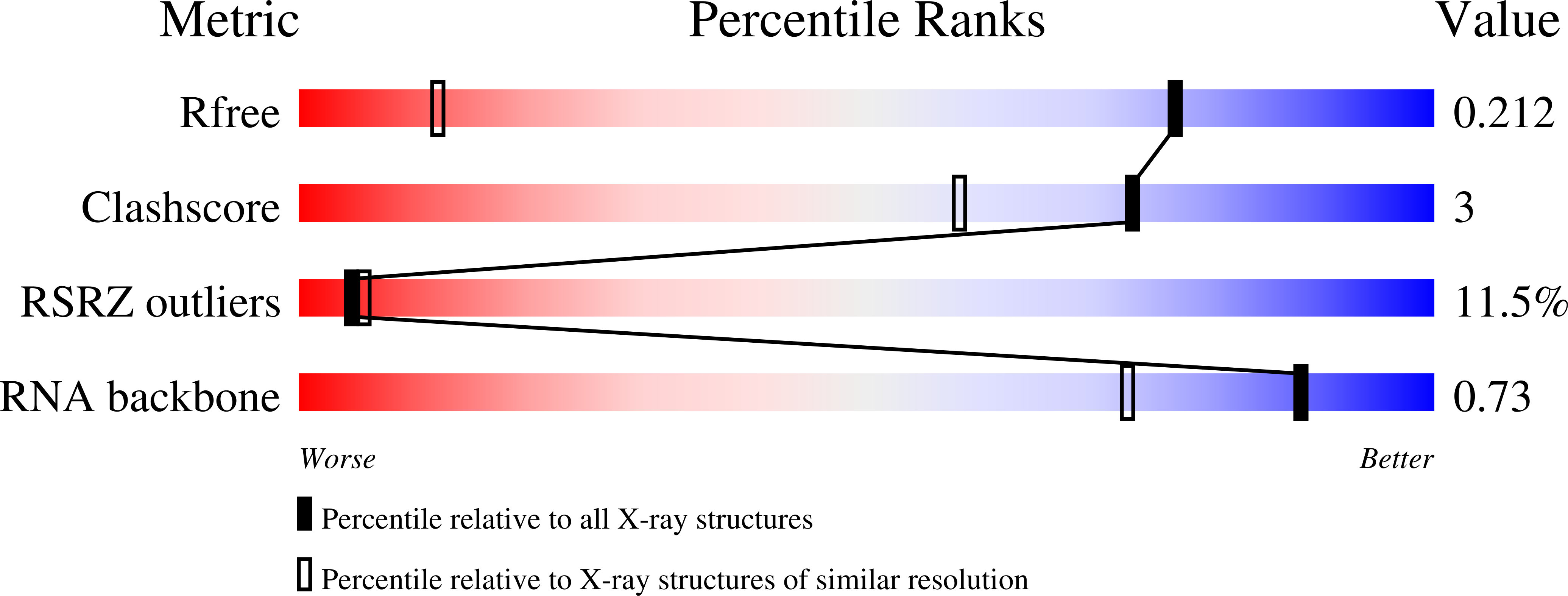Incorporating a Thiophosphate Modification into a Common RNA Tetraloop Motif Causes an Unanticipated Stability Boost.
Pallan, P.S., Lybrand, T.P., Schlegel, M.K., Harp, J.M., Jahns, H., Manoharan, M., Egli, M.(2020) Biochemistry 59: 4627-4637
- PubMed: 33275419
- DOI: https://doi.org/10.1021/acs.biochem.0c00685
- Primary Citation of Related Structures:
7JJD, 7JJE, 7JJF - PubMed Abstract:
GNRA (N = A, C, G, or U; R = A or G) tetraloops are common RNA secondary structural motifs and feature a phosphate stacked atop a nucleobase. The rRNA sarcin/ricin loop (SRL) is capped by G ApGA, and the phosphate p stacks on G . We recently found that regiospecific incorporation of a single dithiophosphate (PS2) but not a monothiophosphate (PSO) instead of phosphate in the backbone of RNA aptamers dramatically increases the binding affinity for their targets. In the RNA:thrombin complex, the key contribution to the 1000-fold tighter binding stems from an edge-on contact between PS2 and a phenylalanine ring. Here we investigated the consequences of replacing the SRL phosphate engaged in a face-on interaction with guanine with either PS2 or PSO for stability. We found that PS2···G and R p-PSO···G contacts stabilize modified SRLs compared to the parent loop to unexpected levels: up to 6.3 °C in melting temperature T m and -4.7 kcal/mol in ΔΔ G °. Crystal structures demonstrate that the vertical distance to guanine for the closest sulfur is just 0.05 Å longer on average compared to that of oxygen despite the larger van der Waals radius of the former (1.80 Å for S vs 1.52 Å for O). The higher stability is enthalpy-based, and the negative charge as assessed by a neutral methylphosphonate modification plays only a minor role. Quantum mechanical/molecular mechanical calculations are supportive of favorable dispersion attraction interactions by sulfur making the dominant contribution. A stacking interaction between phosphate and guanine (SRL) or uracil (U-turn) is also found in newly classified RNA tetraloop families besides GNRA.
Organizational Affiliation:
Alnylam Pharmaceuticals, 300 Third Street, Cambridge, Massachusetts 02142, United States.