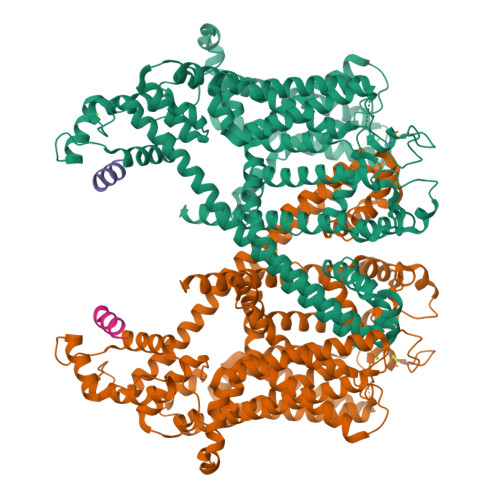
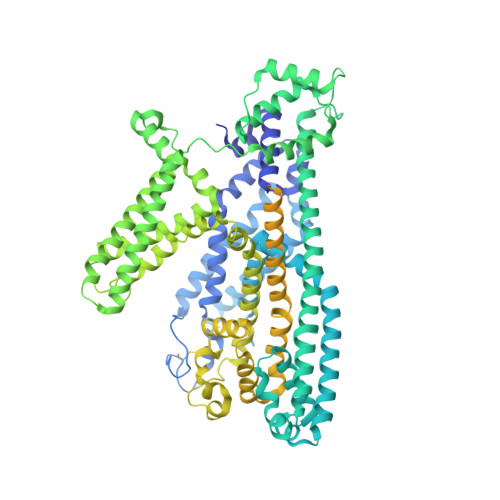
Voltage-gating and cytosolic Ca 2+ activation mechanisms of Arabidopsis two-pore channel AtTPC1.
Ye, F., Xu, L., Li, X., Zeng, W., Gan, N., Zhao, C., Yang, W., Jiang, Y., Guo, J.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 34845029
- DOI: https://doi.org/10.1073/pnas.2113946118
- Primary Citation of Related Structures:
7FHK, 7FHL, 7FHN, 7FHO - PubMed Abstract:
Arabidopsis thaliana two-pore channel AtTPC1 is a voltage-gated, Ca 2+ -modulated, nonselective cation channel that is localized in the vacuolar membrane and responsible for generating slow vacuolar (SV) current. Under depolarizing membrane potential, cytosolic Ca 2+ activates AtTPC1 by binding at the EF-hand domain, whereas luminal Ca 2+ inhibits the channel by stabilizing the voltage-sensing domain II (VSDII) in the resting state. Here, we present 2.8 to 3.3 Å cryoelectron microscopy (cryo-EM) structures of AtTPC1 in two conformations, one in closed conformation with unbound EF-hand domain and resting VSDII and the other in a partially open conformation with Ca 2+ -bound EF-hand domain and activated VSDII. Structural comparison between the two different conformations allows us to elucidate the structural mechanisms of voltage gating, cytosolic Ca 2+ activation, and their coupling in AtTPC1. This study also provides structural insight into the general voltage-gating mechanism among voltage-gated ion channels.
Organizational Affiliation:
Department of Biophysics, and Department of Pathology of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310058, China.