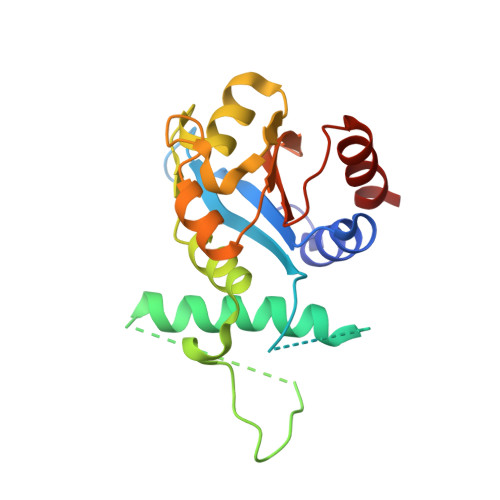
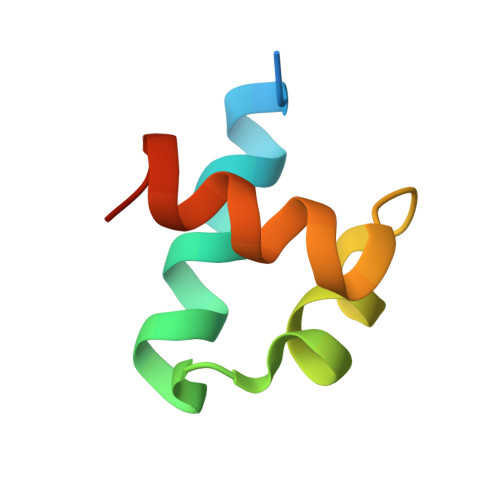
Structural insights into partner selection for MYB and bHLH transcription factor complexes.
Wang, B., Luo, Q., Li, Y., Du, K., Wu, Z., Li, T., Shen, W.H., Huang, C.H., Gan, J., Dong, A.(2021) Nat Plants 8: 1108-1117
- PubMed: 35995835
- DOI: https://doi.org/10.1038/s41477-022-01223-w
- Primary Citation of Related Structures:
7FDL, 7FDM, 7FDN, 7FDO - PubMed Abstract:
MYB and basic helix-loop-helix (bHLH) transcription factors form complexes to regulate diverse metabolic and developmental processes in plants. However, the molecular mechanisms responsible for MYB-bHLH interaction and partner selection remain unclear. Here, we report the crystal structures of three MYB-bHLH complexes (WER-EGL3, CPC-EGL3 and MYB29-MYC3), uncovering two MYB-bHLH interaction modes. WER and CPC are R2R3- and R3-type MYBs, respectively, but interact with EGL3 through their N-terminal R3 domain in a similar mode. A single amino acid of CPC, Met49, is crucial for competition with WER to interact with EGL3. MYB29, a R2R3-type MYB transcription factor, interacts with MYC3 by its C-terminal MYC-interaction motif. The WER-EGL3 and MYB29-MYC3 binding modes are widely applied among MYB-bHLH complexes in Arabidopsis and evolve independently in plants.
Organizational Affiliation:
State Key Laboratory of Genetic Engineering, Collaborative Innovation Center for Genetics and Development, Department of Biochemistry and Biophysics, Institute of Plant Biology, Fudan University, Shanghai, P.R. China.