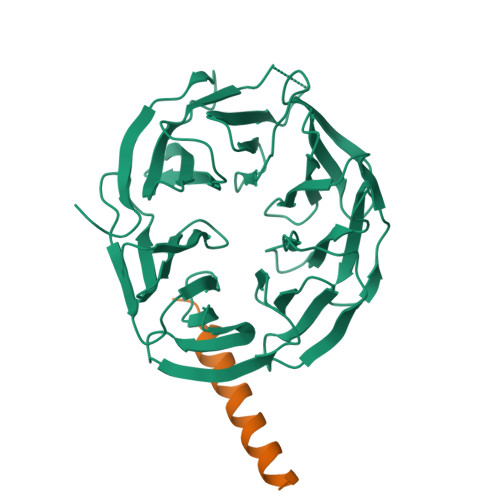
ATG16L1 adopts a dual-binding site mode to interact with WIPI2b in autophagy.
Gong, X., Wang, Y., Tang, Y., Wang, Y., Zhang, M., Li, M., Zhang, Y., Pan, L.(2023) Sci Adv 9: eadf0824-eadf0824
- PubMed: 36857448
- DOI: https://doi.org/10.1126/sciadv.adf0824
- Primary Citation of Related Structures:
7F69, 7XFR - PubMed Abstract:
Macroautophagy plays crucial roles in the regulation of cellular physiology and requires de novo synthesis of double-membrane autophagosomes, which relies on a specific interaction between autophagy-related 16L1 (ATG16L1) and WD repeat domain phosphoinositide-interacting protein 2b (WIPI2b). However, the molecular mechanism governing the interaction of ATG16L1 with WIPI2b remains elusive. Here, we find that ATG16L1 has two distinct binding sites for interacting with WIPI2b, the previously reported WIPI2b-binding site (WBS1) and the previously unidentified site (WBS2). We determine the crystal structures of WIPI2b with ATG16L1 WBS1 and WBS2, respectively, and elucidate the molecular mechanism underpinning the recruitment of ATG16L1 by WIPI2b. Moreover, we uncover that ATG16L1 WBS2 and its binding mode with WIPI2b is well conserved from yeast to mammals, unlike ATG16L1 WBS1. Last, our cell-based functional assays demonstrate that both ATG16L1 WBS1 and WBS2 are required for the effective autophagic flux. In conclusion, our findings provide mechanistic insights into the key ATG16L1/WIPI2b interaction in autophagy.
Organizational Affiliation:
State Key Laboratory of Bio-Organic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200032, China.