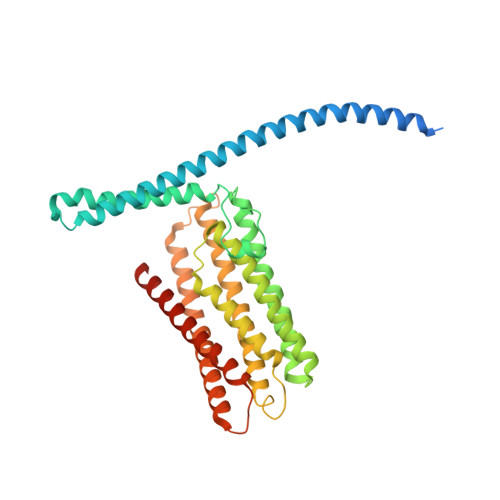
TMEM120A contains a specific coenzyme A-binding site and might not mediate poking- or stretch-induced channel activities in cells.
Rong, Y., Jiang, J., Gao, Y., Guo, J., Song, D., Liu, W., Zhang, M., Zhao, Y., Xiao, B., Liu, Z.(2021) Elife 10
- PubMed: 34409941
- DOI: https://doi.org/10.7554/eLife.71474
- Primary Citation of Related Structures:
7F3T, 7F3U - PubMed Abstract:
TMEM120A, a member of the transmembrane protein 120 (TMEM120) family, has a pivotal function in adipocyte differentiation and metabolism, and may also contribute to sensing mechanical pain by functioning as an ion channel named TACAN. Here we report that expression of TMEM120A is not sufficient in mediating poking- or stretch-induced currents in cells and have solved cryo-electron microscopy (cryo-EM) structures of human TMEM120A ( Hs TMEM120A) in complex with an endogenous metabolic cofactor (coenzyme A, CoASH) and in the apo form. Hs TMEM120A forms a symmetrical homodimer with each monomer containing an amino-terminal coiled-coil motif followed by a transmembrane domain with six membrane-spanning helices. Within the transmembrane domain, a CoASH molecule is hosted in a deep cavity and forms specific interactions with nearby amino acid residues. Mutation of a central tryptophan residue involved in binding CoASH dramatically reduced the binding affinity of Hs TMEM120A with CoASH. Hs TMEM120A exhibits distinct conformations at the states with or without CoASH bound. Our results suggest that TMEM120A may have alternative functional roles potentially involved in CoASH transport, sensing, or metabolism.
Organizational Affiliation:
National Laboratory of Biomacromolecules, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China.