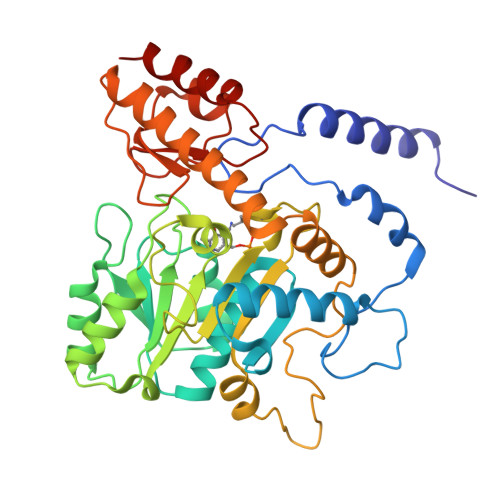
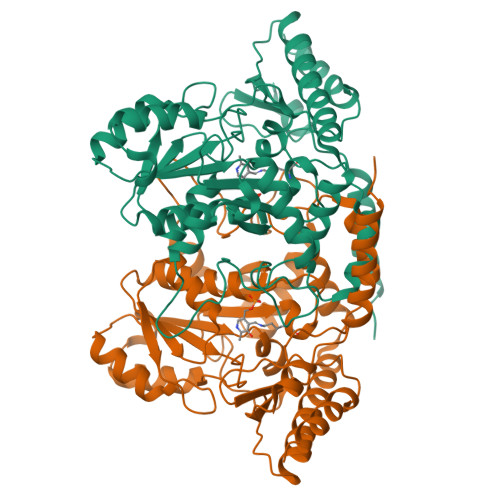
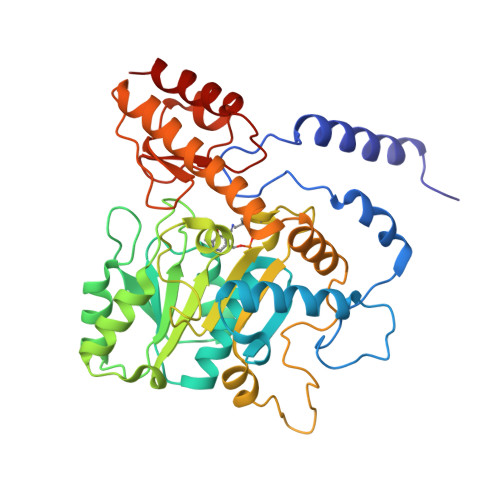
Structural insights into the enhanced thermostability of cysteine substitution mutants of L-histidine decarboxylase from Photobacterium phosphoreum.
Oda, Y., Nakata, K., Miyano, H., Mizukoshi, T., Yamaguchi, H., Kashiwagi, T.(2022) J Biochem 171: 31-40
- PubMed: 34622278
- DOI: https://doi.org/10.1093/jb/mvab103
- Primary Citation of Related Structures:
7ERU, 7ERV - PubMed Abstract:
Enzymatic amino acid assays are important in physiological research and clinical diagnostics because abnormal amino acid concentrations in biofluids are associated with various diseases. L-histidine decarboxylase from Photobacterium phosphoreum (PpHDC) is a pyridoxal 5'-phosphate-dependent enzyme and a candidate for use in an L-histidine quantitation assay. Previous cysteine substitution experiments demonstrated that the PpHDC C57S mutant displayed improved long-term storage stability and thermostability when compared with those of the wild-type enzyme. In this study, combinational mutation experiments of single cysteine substitution mutants of PpHDC were performed, revealing that the PpHDC C57S/C101V/C282V mutant possessed the highest thermostability. The stabilizing mechanism of these mutations was elucidated by solving the structures of PpHDC C57S and C57S/C101V/C282V mutants by X-ray crystallography. In the crystal structures, two symmetry-related PpHDC molecules form a domain-swapped homodimer. The side chain of S57 is solvent exposed in the structure, indicating that the C57S mutation eliminates chemical oxidation or disulfide bond formation with a free thiol group, thereby providing greater stability. Residues 101 and 282 form hydrophobic interactions with neighboring hydrophobic residues. Mutations C101V and C282V enhanced thermostability of PpHDC by filling a cavity present in the hydrophobic core (C101V) and increasing hydrophobic interactions.
Organizational Affiliation:
Ajinomoto Co., Inc., Kawasaki, Kanagawa 210-8681, Japan.