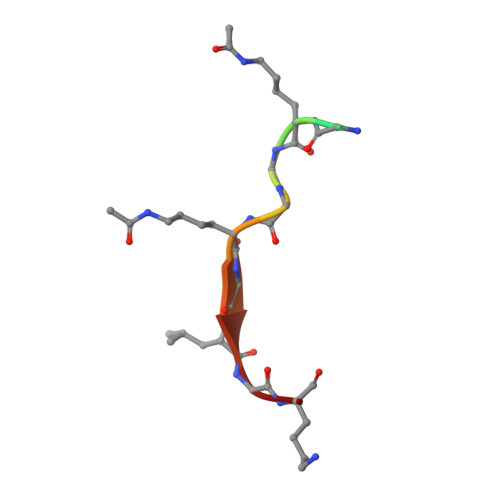
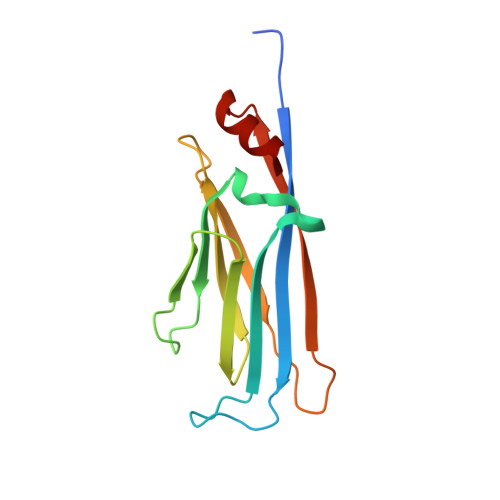
Elucidation of binding preferences of YEATS domains to site-specific acetylated nucleosome core particles.
Kikuchi, M., Morita, S., Goto, M., Wakamori, M., Katsura, K., Hanada, K., Shirouzu, M., Umehara, T.(2022) J Biological Chem 298: 102164-102164
- PubMed: 35732209
- DOI: https://doi.org/10.1016/j.jbc.2022.102164
- Primary Citation of Related Structures:
7EIC, 7EID, 7EIE - PubMed Abstract:
Acetylated lysine residues (Kac) in histones are recognized by epigenetic reader proteins, such as Yaf9, ENL, AF9, Taf14, and Sas5 (YEATS) domain-containing proteins. Human YEATS domains bind to the acetylated N-terminal tail of histone H3; however, their Kac-binding preferences at the level of the nucleosome are unknown. Through genetic code reprogramming, here, we established a nucleosome core particle (NCP) array containing histones that were acetylated at specific residues and used it to compare the Kac-binding preferences of human YEATS domains. We found that AF9-YEATS showed basal binding to the unmodified NCP and that it bound stronger to the NCP containing a single acetylation at one of K4, K9, K14, or K27 of H3, or to histone H4 multi-acetylated between K5 and K16. Crystal structures of AF9-YEATS in complex with an H4 peptide diacetylated either at K5/K8 or K8/K12 revealed that the aromatic cage of the YEATS domain recognized the acetylated K8 residue. Interestingly, E57 and D103 of AF9, both located outside of the aromatic cage, were shown to interact with acetylated K5 and K12 of H4, respectively, consistent with the increase in AF9-YEATS binding to the H4K8-acetylated NCP upon additional acetylation at K5 or K12. Finally, we show that a mutation of E57 to alanine in AF9-YEATS reduced the binding affinity for H4 multiacetylated NCPs containing H4K5ac. Our data suggest that the Kac-binding affinity of AF9-YEATS increases additively with the number of Kac in the histone tail.
Organizational Affiliation:
RIKEN Center for Biosystems Dynamics Research, Tsurumi, Yokohama, Japan.