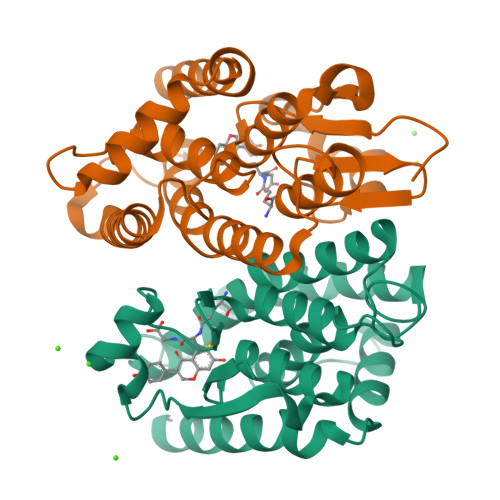
Molecular action of larvicidal flavonoids on ecdysteroidogenic glutathione S-transferase Noppera-bo in Aedes aegypti.
Inaba, K., Ebihara, K., Senda, M., Yoshino, R., Sakuma, C., Koiwai, K., Takaya, D., Watanabe, C., Watanabe, A., Kawashima, Y., Fukuzawa, K., Imamura, R., Kojima, H., Okabe, T., Uemura, N., Kasai, S., Kanuka, H., Nishimura, T., Watanabe, K., Inoue, H., Fujikawa, Y., Honma, T., Hirokawa, T., Senda, T., Niwa, R.(2022) BMC Biol 20: 43-43
- PubMed: 35172816
- DOI: https://doi.org/10.1186/s12915-022-01233-2
- Primary Citation of Related Structures:
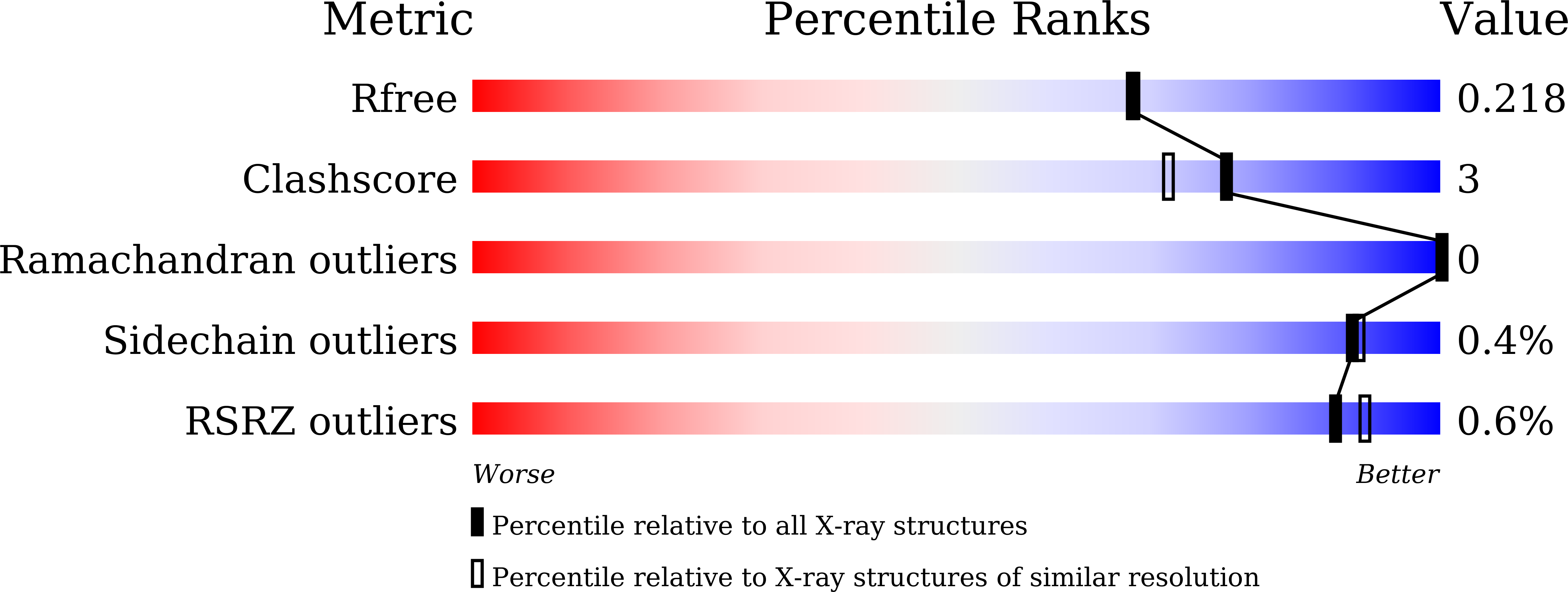
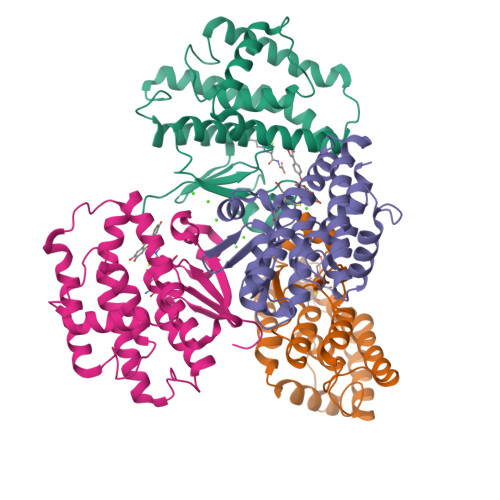
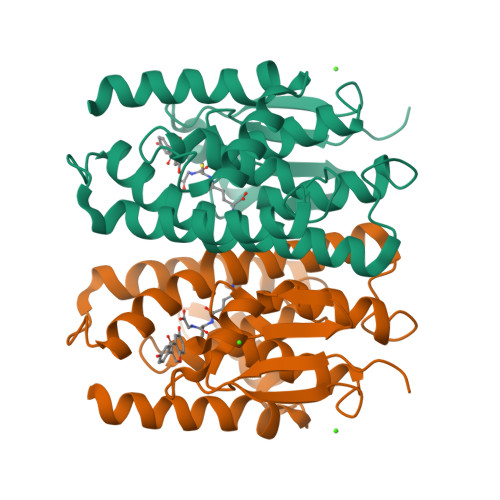
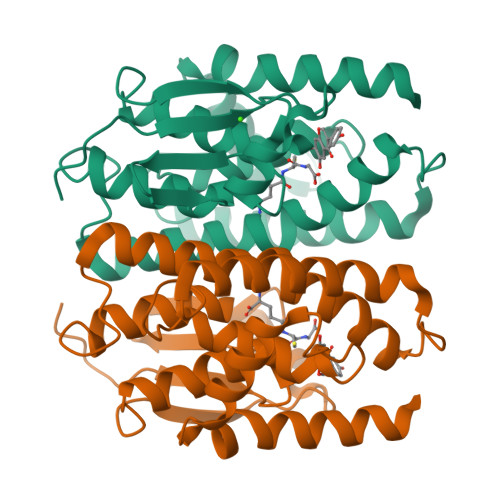
7EBT, 7EBU, 7EBV, 7EBW - PubMed Abstract:
Mosquito control is a crucial global issue for protecting the human community from mosquito-borne diseases. There is an urgent need for the development of selective and safe reagents for mosquito control. Flavonoids, a group of chemical substances with variable phenolic structures, such as daidzein, have been suggested as potential mosquito larvicides with less risk to the environment. However, the mode of mosquito larvicidal action of flavonoids has not been elucidated. Here, we report that several flavonoids, including daidzein, inhibit the activity of glutathione S-transferase Noppera-bo (Nobo), an enzyme used for the biosynthesis of the insect steroid hormone ecdysone, in the yellow fever mosquito Aedes aegypti. The crystal structure of the Nobo protein of Ae. aegypti (AeNobo) complexed with the flavonoids and its molecular dynamics simulation revealed that Glu113 forms a hydrogen bond with the flavonoid inhibitors. Consistent with this observation, substitution of Glu113 with Ala drastically reduced the inhibitory activity of the flavonoids against AeNobo. Among the identified flavonoid-type inhibitors, desmethylglycitein (4',6,7-trihydroxyisoflavone) exhibited the highest inhibitory activity in vitro. Moreover, the inhibitory activities of the flavonoids correlated with the larvicidal activity, as desmethylglycitein suppressed Ae. aegypti larval development more efficiently than daidzein. Our study demonstrates the mode of action of flavonoids on the Ae. aegypti Nobo protein at the atomic, enzymatic, and organismal levels.
Organizational Affiliation:
Graduate School of Life and Environmental Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki, 305-8572, Japan.