Structures of PKA-phospholamban complexes reveal a mechanism of familial dilated cardiomyopathy.
Qin, J., Zhang, J., Lin, L., Haji-Ghassemi, O., Lin, Z., Woycechowsky, K.J., Van Petegem, F., Zhang, Y., Yuchi, Z.(2022) Elife 11
- PubMed: 35297759
- DOI: https://doi.org/10.7554/eLife.75346
- Primary Citation of Related Structures:
7E0Z, 7E11, 7E12 - PubMed Abstract:
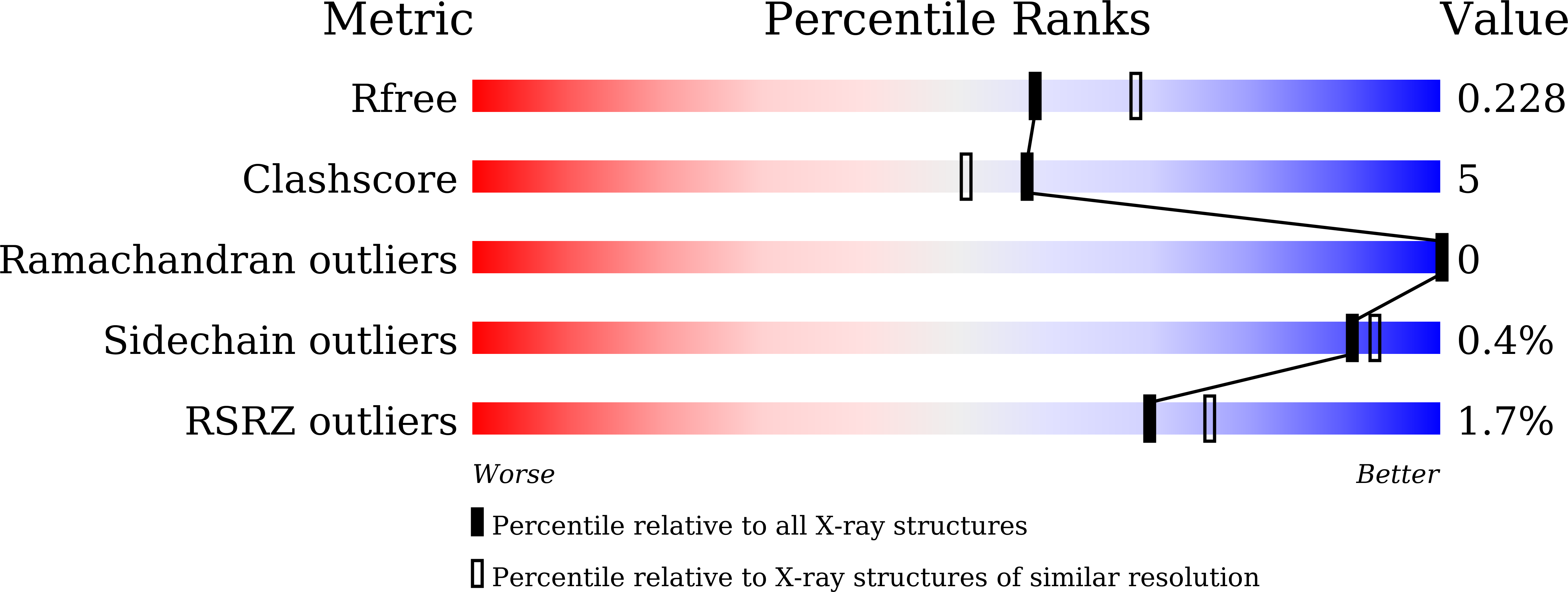
Several mutations identified in phospholamban (PLN) have been linked to familial dilated cardiomyopathy (DCM) and heart failure, yet the underlying molecular mechanism remains controversial. PLN interacts with sarco/endoplasmic reticulum Ca 2+ -ATPase (SERCA) and regulates calcium uptake, which is modulated by the protein kinase A (PKA)-dependent phosphorylation of PLN during the fight-or-flight response. Here, we present the crystal structures of the catalytic domain of mouse PKA in complex with wild-type and DCM-mutant PLNs. Our structures, combined with the results from other biophysical and biochemical assays, reveal a common disease mechanism: the mutations in PLN reduce its phosphorylation level by changing its conformation and weakening its interactions with PKA. In addition, we demonstrate that another more ubiquitous SERCA-regulatory peptide, called another-regulin (ALN), shares a similar mechanism mediated by PKA in regulating SERCA activity.
Organizational Affiliation:
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency; Collaborative Innovation Center of Chemical Science and Engineering; School of Pharmaceutical Science and Technology, Tianjin University, Tianjin, China.