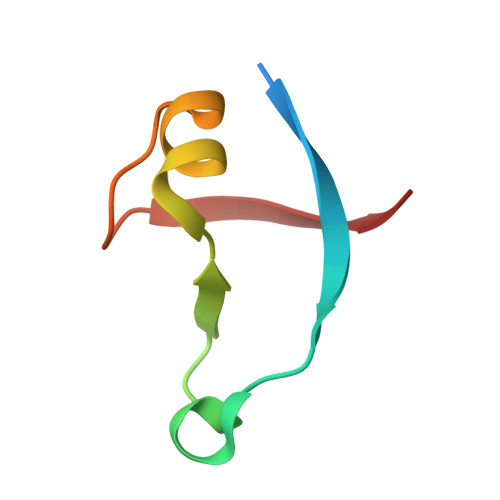
Seven Amino Acid Types Suffice to Create the Core Fold of RNA Polymerase.
Yagi, S., Padhi, A.K., Vucinic, J., Barbe, S., Schiex, T., Nakagawa, R., Simoncini, D., Zhang, K.Y.J., Tagami, S.(2021) J Am Chem Soc 143: 15998-16006
- PubMed: 34559526
- DOI: https://doi.org/10.1021/jacs.1c05367
- Primary Citation of Related Structures:
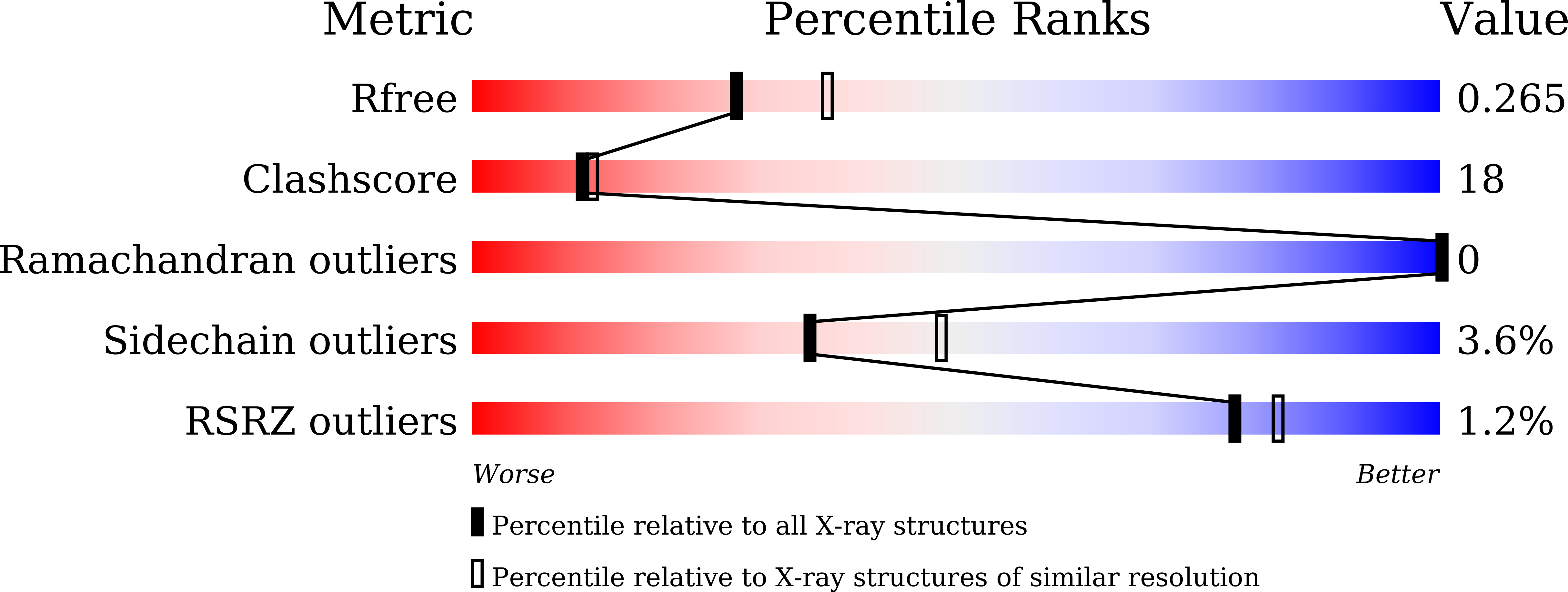
7DBO, 7DG7, 7DG9, 7DI0, 7DI1, 7DU6, 7DU7, 7DVC, 7DVF, 7DVH, 7DWW, 7DXR, 7DXS, 7DXT, 7DXU, 7DXV, 7DXW, 7DXX, 7DXY, 7DXZ, 7DYC - PubMed Abstract:
The extant complex proteins must have evolved from ancient short and simple ancestors. The double-ψ β-barrel (DPBB) is one of the oldest protein folds and conserved in various fundamental enzymes, such as the core domain of RNA polymerase. Here, by reverse engineering a modern DPBB domain, we reconstructed its plausible evolutionary pathway started by "interlacing homodimerization" of a half-size peptide, followed by gene duplication and fusion. Furthermore, by simplifying the amino acid repertoire of the peptide, we successfully created the DPBB fold with only seven amino acid types (Ala, Asp, Glu, Gly, Lys, Arg, and Val), which can be coded by only GNN and ARR (R = A or G) codons in the modern translation system. Thus, the DPBB fold could have been materialized by the early translation system and genetic code.
Organizational Affiliation:
RIKEN Center for Biosystems Dynamics Research, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan.