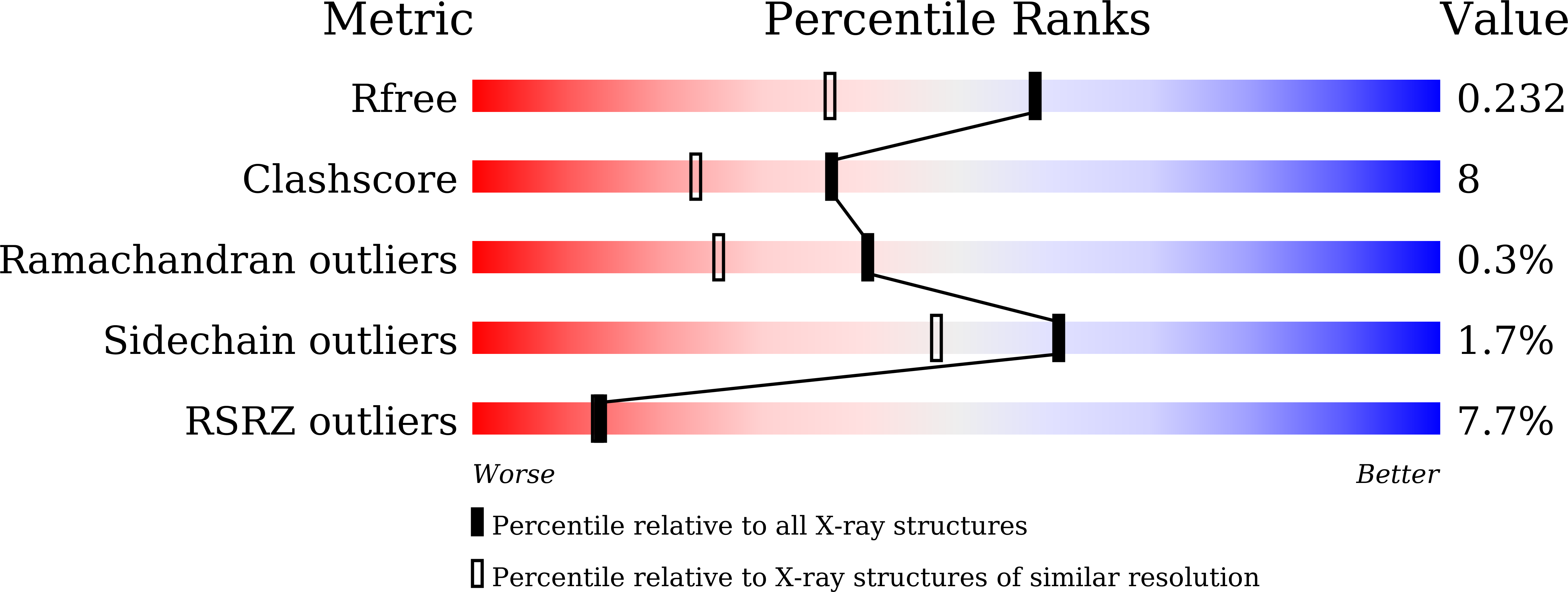
Structure of a ternary complex of lactoperoxidase with iodide and hydrogen peroxide at 1.77 angstrom resolution.
Singh, P.K., Sharma, P., Bhushan, A., Kaur, P., Sharma, S., Singh, T.P.(2021) J Inorg Biochem 220: 111461-111461
- PubMed: 33882424
- DOI: https://doi.org/10.1016/j.jinorgbio.2021.111461
- Primary Citation of Related Structures:
7DLQ, 7DN6, 7DN7 - PubMed Abstract:
Lactoperoxidase (LPO) is a mammalian heme peroxidase which catalyzes the conversion of thiocyanate (SCN¯) and iodide (I - ) by hydrogen peroxide (H 2 O 2 ) into antimicrobial hypothiocyanite (OSCN¯) and hypoiodite (IO - ). The prosthetic heme group is covalently attached to LPO through two ester linkages involving conserved glutamate and aspartate residues. On the proximal side, His351 is coordinated to heme iron while His 109 is located in the substrate binding site on the distal heme side. We report here the first structure of the ternary complex of LPO with iodide (I - ) and H 2 O 2 at 1.77 Å resolution. LPO was crystallized with ammonium iodide and the crystals were soaked in the reservoir solution containing H 2 O 2. Structure determination showed the presence of an iodide ion and a H 2 O 2 molecule in the substrate binding site. The iodide ion occupied the position which is stabilized by the interactions with heme moiety, His109, Arg255 and Glu258 while H 2 O 2 was held between the heme iron and His109. The presence of I - in the distal heme cavity seems to screen the positive charge of Arg255 thus suppressing the proton transfer from H 2 O 2 to His109. This prevents compound I formation and allows trapping of a stable enzyme-substrate (LPO-I - -H 2 O 2) ternary complex. This stable geometrical arrangement of H 2 O 2 in the distal heme cavity of LPO is similar to that of H 2 O 2 in the structure of the transient intermediate of the palm tree heme peroxidase. The biochemical studies showed that the catalytic activity of LPO decreased when the samples of LPO were preincubated with ammonium iodide.
Organizational Affiliation:
Department of Biophysics, All India Institute of Medical Sciences, New Delhi, India.