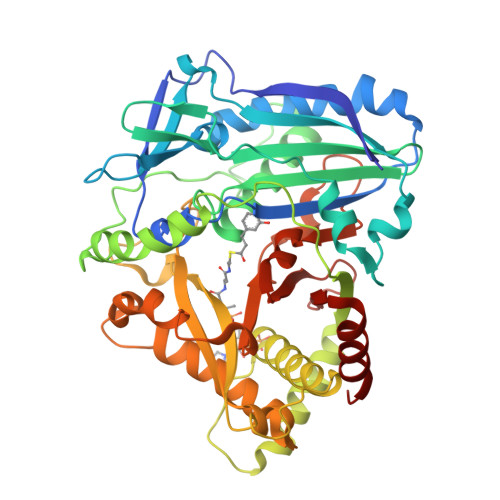
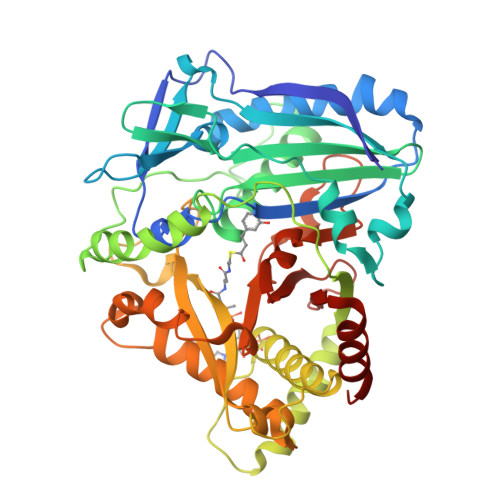
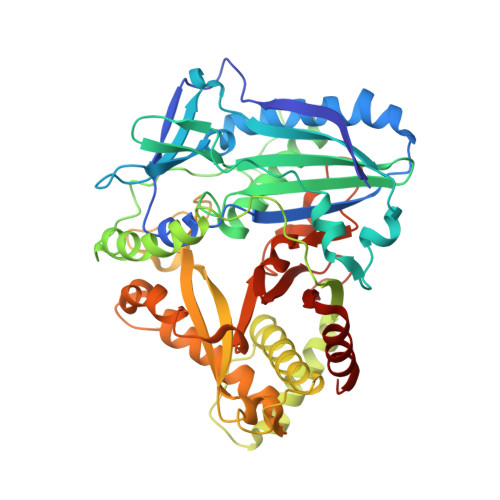
Anthocyanin 5,3'-aromatic acyltransferase from Gentiana triflora, a structural insight into biosynthesis of a blue anthocyanin.
Murayama, K., Kato-Murayama, M., Sato, T., Hosaka, T., Ishiguro, K., Mizuno, T., Kitao, K., Honma, T., Yokoyama, S., Tanaka, Y., Shirouzu, M.(2021) Phytochemistry 186: 112727-112727
- PubMed: 33743393
- DOI: https://doi.org/10.1016/j.phytochem.2021.112727
- Primary Citation of Related Structures:
7DEV, 7DEX - PubMed Abstract:
The acylation of anthocyanins contributes to their structural diversity. Aromatic acylation is responsible for the blue color of anthocyanins and certain flowers. Aromatic acyltransferase from Gentiana triflora Pall. (Gentianaceae) (Gt5,3'AT) catalyzes the acylation of glucosyl moieties at the 5 and 3' positions of anthocyanins. Anthocyanin acyltransferase transfers an acyl group to a single position, such that Gt5,3'AT possesses a unique enzymatic activity. Structural investigation of this aromatic acyl group transfer is fundamental to understand the molecular mechanism of the acylation of double positions. In this study, structural analyses of Gt5,3'AT were conducted to identify the underlying mechanism. The crystal structure indicated that Gt5,3'AT shares structural similarities with other BAHD family enzymes, consisting of N and C terminal lobes. Structural comparison revealed that acyl group preference (aromatic or aliphatic) for the enzymes was determined by four amino acid positions, which are well conserved in aromatic and aliphatic CoA-binding acyltransferases. Although a complex structure with anthocyanins was not obtained, the binding of delphinidin 3,5,3'-triglucoside to Gt5,3'AT was investigated by evaluating the molecular dynamics. The simulation indicated that acyl transfer by Gt5,3'AT preferentially occurs at the 5-position rather than at the 3'-position, with interacting amino acids that are mainly located in the C-terminal lobe. Subsequent assays of chimeric enzymes (exchange of the N-terminal lobe and the C-terminal lobe between Gt5,3'AT and lisianthus anthocyanin 5AT) demonstrated that acyl transfer selectivity may be caused by the C-terminal lobe.
Organizational Affiliation:
Division of Biomedical Measurements and Diagnostics, Graduate School of Biomedical Engineering, Tohoku University, Sendai, 980-8575, Japan; Laboratory for Protein Functional and Structural Biology, RIKEN Center for Biosystems Dynamics Research, Yokohama, 230-0045, Japan.