Exploitation of Elevated Extracellular ATP to Specifically Direct Antibody to Tumor Microenvironment.
Mimoto, F., Tatsumi, K., Shimizu, S., Kadono, S., Haraya, K., Nagayasu, M., Suzuki, Y., Fujii, E., Kamimura, M., Hayasaka, A., Kawauchi, H., Ohara, K., Matsushita, M., Baba, T., Susumu, H., Sakashita, T., Muraoka, T., Aso, K., Katada, H., Tanaka, E., Nakagawa, K., Hasegawa, M., Ayabe, M., Yamamoto, T., Tanba, S., Ishiguro, T., Kamikawa, T., Nambu, T., Kibayashi, T., Azuma, Y., Tomii, Y., Kato, A., Ozeki, K., Murao, N., Endo, M., Kikuta, J., Kamata-Sakurai, M., Ishii, M., Hattori, K., Igawa, T.(2020) Cell Rep 33: 108542-108542
- PubMed: 33357423
- DOI: https://doi.org/10.1016/j.celrep.2020.108542
- Primary Citation of Related Structures:
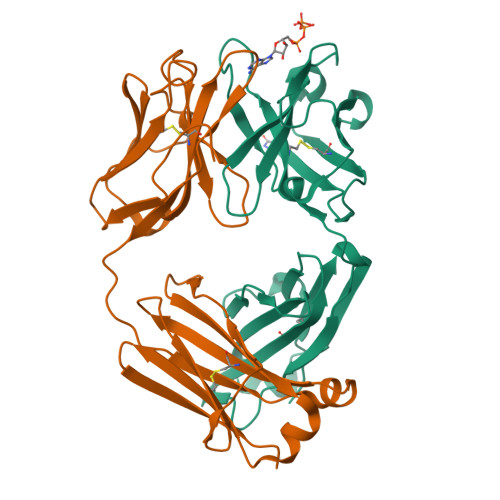
7DC7, 7DC8 - PubMed Abstract:
The extracellular adenosine triphosphate (ATP) concentration is highly elevated in the tumor microenvironment (TME) and remains tightly regulated in normal tissues. Using phage display technology, we establish a method to identify an antibody that can bind to an antigen only in the presence of ATP. Crystallography analysis reveals that ATP bound in between the antibody-antigen interface serves as a switch for antigen binding. In a transgenic mouse model overexpressing the antigen systemically, the ATP switch antibody binds to the antigen in tumors with minimal binding in normal tissues and plasma and inhibits tumor growth. Thus, we demonstrate that elevated extracellular ATP concentration can be exploited to specifically target the TME, giving therapeutic antibodies the ability to overcome on-target off-tumor toxicity.
Organizational Affiliation:
Chugai Pharmabody Research Pte. Ltd., 3 Biopolis Drive, #07 - 11 to 16, Synapse, 138623, Singapore. Electronic address: mimoto-f@chugai-pharmabody.com.