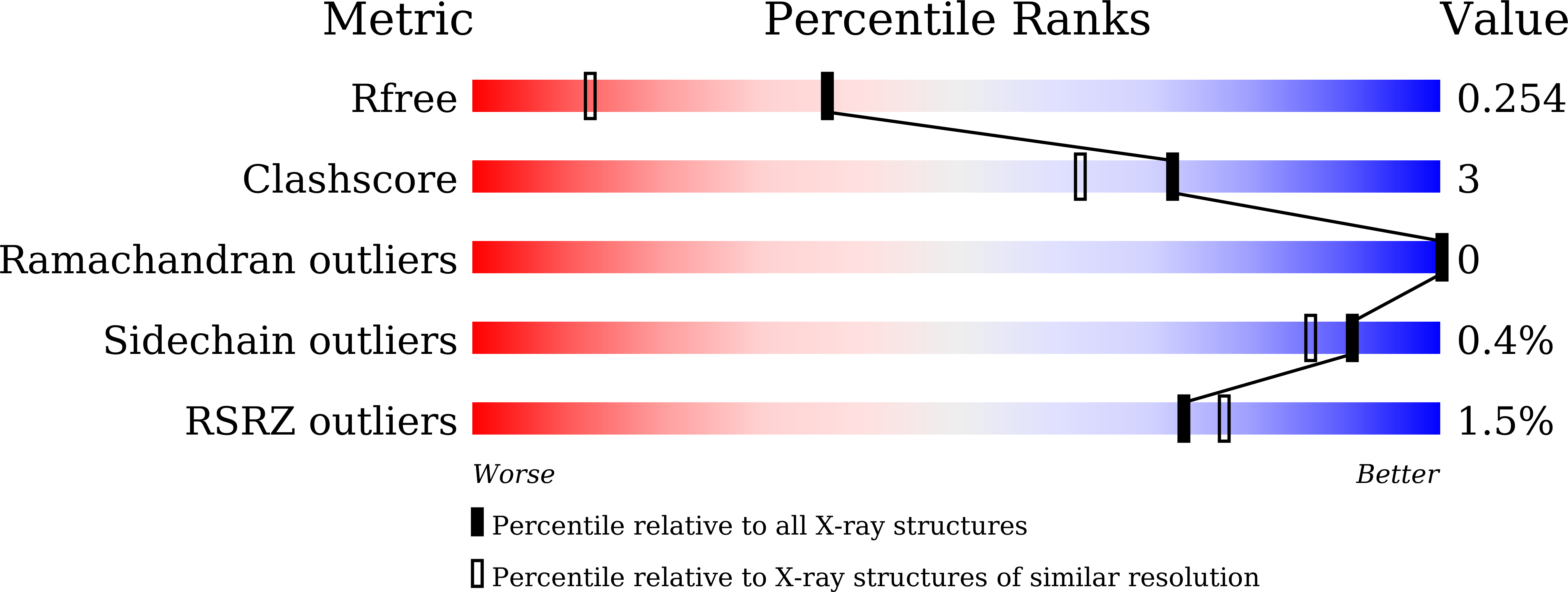
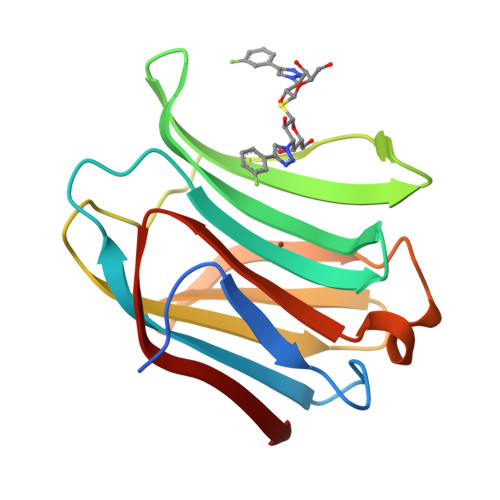
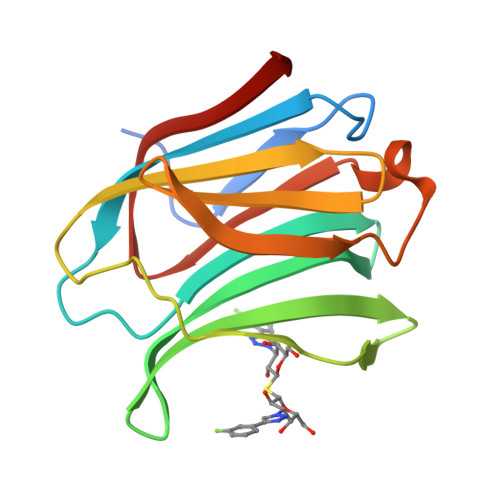
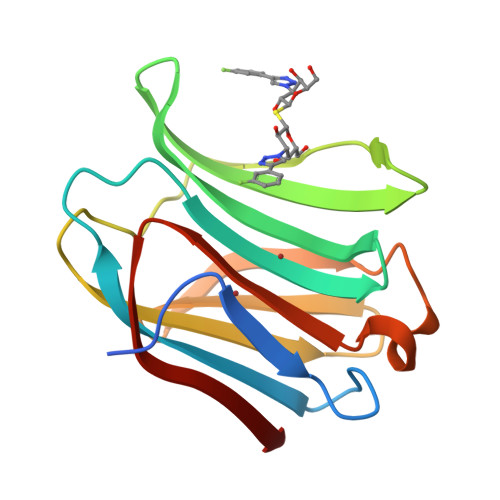
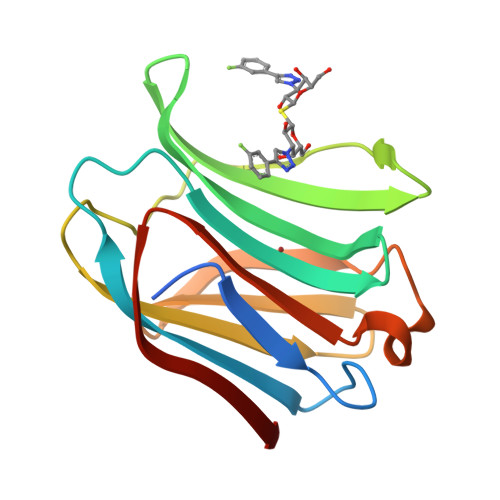
Molecular mechanism of interspecies differences in the binding affinity of TD139 to Galectin-3.
Kumar, A., Paul, M., Panda, M., Jayaram, S., Kalidindi, N., Sale, H., Vetrichelvan, M., Gupta, A., Mathur, A., Beno, B., Regueiro-Ren, A., Cheng, D., Ramarao, M., Ghosh, K.(2021) Glycobiology 31: 1390-1400
- PubMed: 34228782
- DOI: https://doi.org/10.1093/glycob/cwab072
- Primary Citation of Related Structures:
7CXA, 7CXB, 7CXC, 7CXD - PubMed Abstract:
Galectin-3 (Gal-3), a β-galactoside-binding lectin, has been implicated in a plethora of pathological disorders including fibrosis, inflammation, cancer and metabolic diseases. TD139-a thio-digalactoside inhibitor developed by Galecto Biotech as a potential therapeutic for idiopathic pulmonary fibrosis-is the most advanced small-molecule Gal-3 inhibitor in clinical studies. It binds to human Gal-3 with high affinity but has lower affinity towards mouse and rat homologs, which is also manifested in the differential inhibition of Gal-3 function. Using biophysical methods and high-resolution X-ray co-crystal structures of TD139 and Gal-3 proteins, we demonstrate that a single amino acid change corresponding to A146 in human Gal-3 is sufficient for the observed reduction in the binding affinity of TD139 in rodents. Site-directed mutagenesis of A146V (in human Gal-3) and V160A (in mouse Gal-3) was sufficient to interchange the affinities, mainly by affecting the off rates of the inhibitor binding. In addition, molecular dynamics simulations of both wild-type and mutant structures revealed the sustained favorable noncovalent interactions between the fluorophenyl ring and the active site A146 (human Gal-3 and mouse V160A) that corroborate the finding from biophysical studies. Current findings have ramifications in the context of optimization of drug candidates against Gal-3.
Organizational Affiliation:
Discovery Biology and Translational Medicine, Biocon Bristol-Myers Squibb R&D Center, Bristol-Myers Squibb India Pvt. Ltd, Bangalore 560099, India.