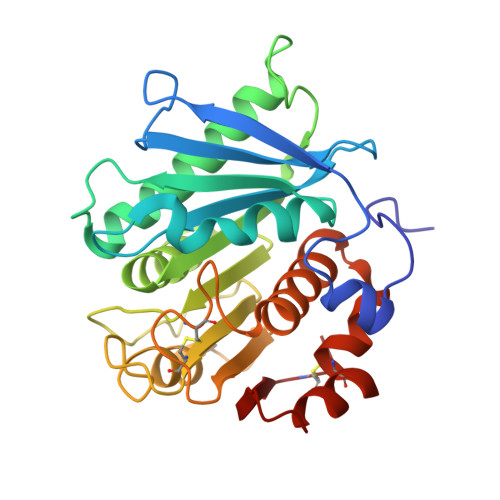
General features to enhance enzymatic activity of poly(ethylene terephthalate) hydrolysis.
Che, C.C., Han, X., Li, X., Jiang, P., Niu, D., Ma, L., Liu, W., Li, S., Qu, Y., Hu, H., Min, J., Yang, Y., Zhang, L., Zeng, W., Huang, J.W., Dai, L., Guo, R.T.(2021) Nat Catal 4: 425-430