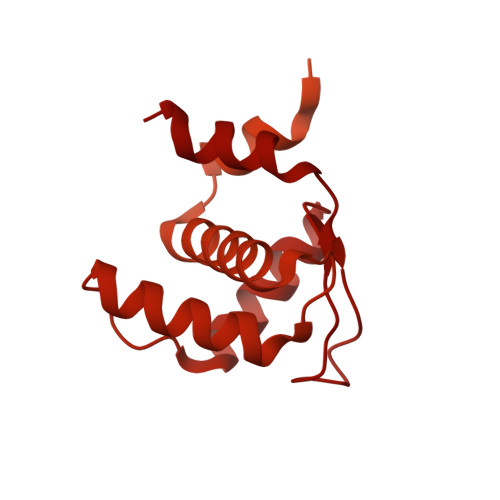
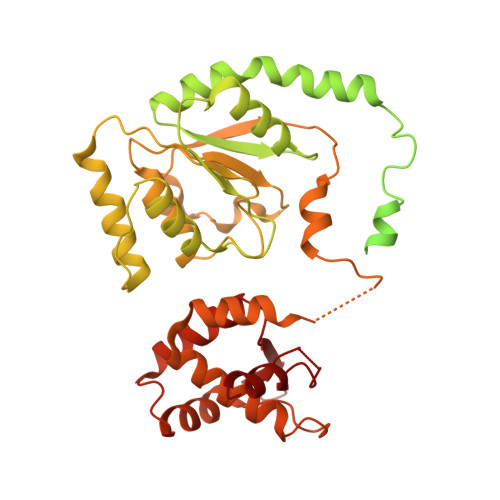
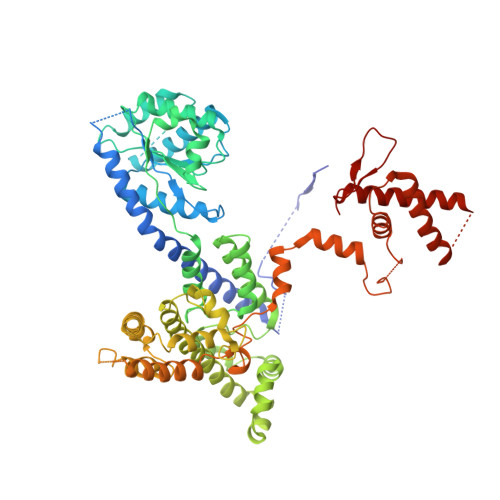
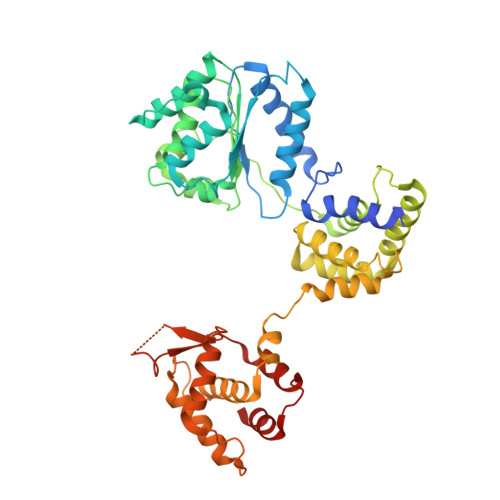
Structural insight into the assembly and conformational activation of human origin recognition complex.
Cheng, J., Li, N., Wang, X., Hu, J., Zhai, Y., Gao, N.(2020) Cell Discov 6: 88-88
- PubMed: 33298899
- DOI: https://doi.org/10.1038/s41421-020-00232-3
- Primary Citation of Related Structures:
7CTE, 7CTF, 7CTG - PubMed Abstract:
The function of the origin recognition complex (ORC) in DNA replication is highly conserved in recognizing and marking the initiation sites. The detailed molecular mechanisms by which human ORC is reconfigured into a state competent for origin association remain largely unknown. Here, we present structural characterizations of human ORC1-5 and ORC2-5 assemblies. ORC2-5 exhibits a tightly autoinhibited conformation with the winged-helix domain of ORC2 completely blocking the central DNA-binding channel. The binding of ORC1 partially relieves the autoinhibitory effect of ORC2-5 through remodeling ORC2-WHD, which makes ORC2-WHD away from the central channel creating a still autoinhibited but more dynamic structure. In particular, the AAA+ domain of ORC1 is highly flexible to sample a variety of conformations from inactive to potentially active states. These results provide insights into the detailed mechanisms regulating the autoinhibition of human ORC and its subsequent activation for DNA binding.
Organizational Affiliation:
State Key Laboratory of Membrane Biology, School of Life Sciences, Tsinghua University, Beijing 100084, China.