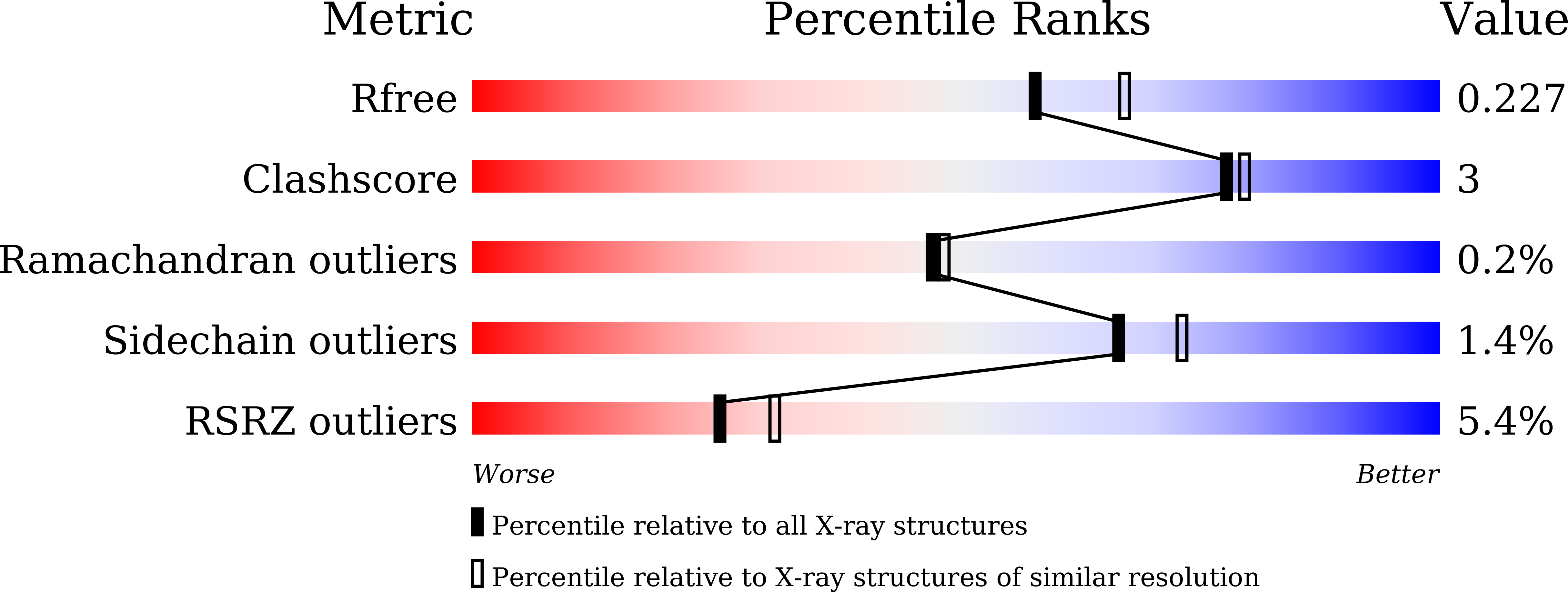
Structural snapshots of CmoB in various states during wobble uridine modification of tRNA.
Jeong, S., Kim, J.(2021) Biochem Biophys Res Commun 534: 604-609
- PubMed: 33213836
- DOI: https://doi.org/10.1016/j.bbrc.2020.11.033
- Primary Citation of Related Structures:
7CT8, 7CT9, 7CTA - PubMed Abstract:
CmoB utilizes carboxy-S-adenosyl-l-methionine (CxSAM) to carry out unusual carboxymethyl transfer to form 5-carboxymethoxyuridine (cmo 5 U) of several tRNA species in Gram-negative bacteria. In this report, we present three X-ray crystal structures of CmoB from Vibrio vulnificus representing different states in the course of the reaction pathway; i.e., apo-, substrate-bound, and product-bound forms. Especially, the crystal structure of apo-CmoB unveils a novel open state of the enzyme, capturing unprecedented conformational dynamics around the substrate-binding site. The apo-structure demonstrates that the open conformation favors the release of CxSAM thus representing an inactive form. Our crystal structures of CmoB complexed with CxSAM and S-adenosyl-l-homocysteine (SAH) and combined binding assay results support the proposed mechanism underlying the cofactor selectivity, where CmoB preferentially senses negative charge around amino acid residues Lys-91, Tyr-200, and Arg-315.
Organizational Affiliation:
Department of Chemistry, Gwangju Institute of Science and Technology, Gwangju, 61005, South Korea.