Insights into the structure of mature streptavidin C1 from Streptomyces cinnamonensis reveal the self-binding of the extension C-terminal peptide to biotin-binding sites.
Jeon, B.J., Kim, S., Kim, M.S., Lee, J.H., Kim, B.S., Hwang, K.Y.(2021) IUCrJ 8: 168-177
- PubMed: 33708394
- DOI: https://doi.org/10.1107/S2052252520015675
- Primary Citation of Related Structures:
7CPZ, 7CQ0 - PubMed Abstract:
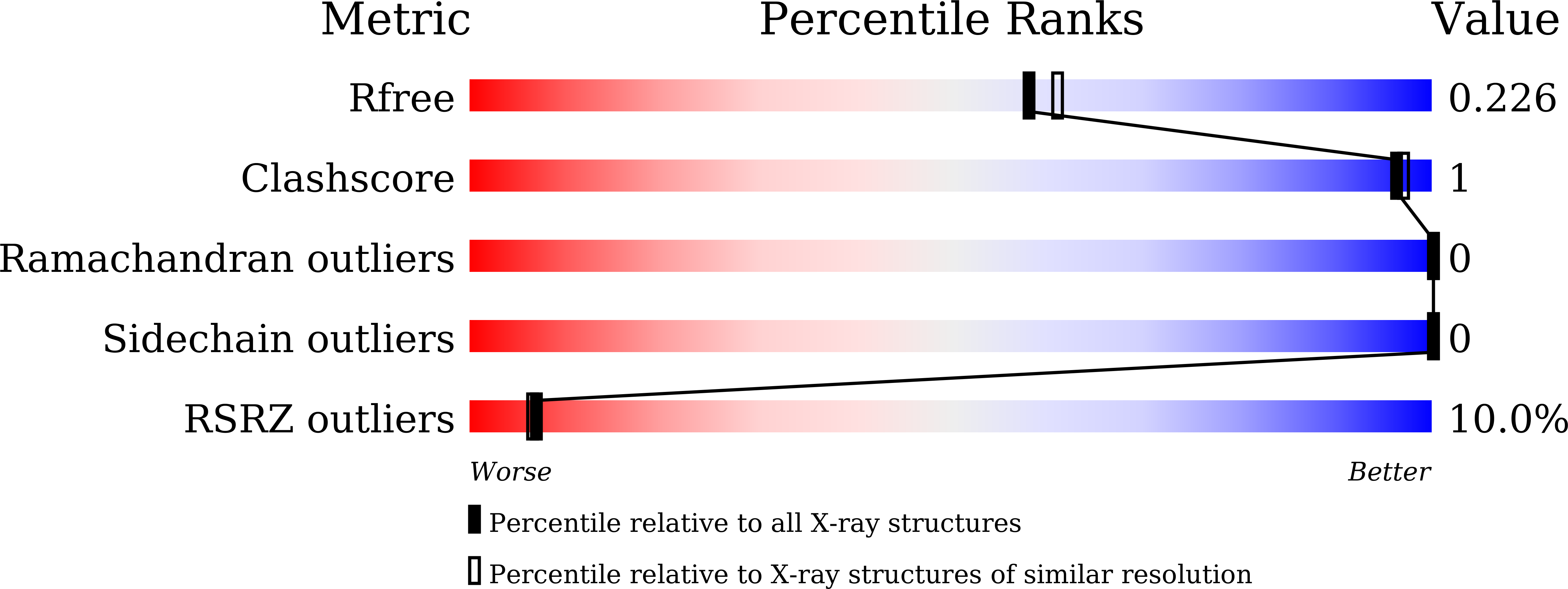
The members of the avidin protein family are well known for their high affinity towards d-biotin and their structural stability. These properties make avidins a valuable tool for various biotechnological applications. In the present study, two avidin-like biotin-binding proteins (named streptavidin C1 and C2) from Streptomyces cinnamonensis were newly identified while exploring antifungal proteins against Fusarium oxysporum f. sp. cucumerinum . Streptavidin C1 reveals a low correlation (a sequence identity of approximately 64%) with all known streptavidins, whereas streptavidin C2 shares a sequence identity of approximately 94% with other streptavidins. Here, the crystal structures of streptavidin C1 in the mature form and in complex with biotin at 2.1 and 2.5 Å resolution, respectively, were assessed. The overall structures present similar tetrameric features with D 2 symmetry to other (strept)avidin structures. Interestingly, the long C-terminal region comprises a short α-helix (C-Lid; residues 169-179) and an extension C-terminal peptide (ECP; residues 180-191) which stretches into the biotin-binding sites of the same monomer. This ECP sequence (- 180 VTSANPPAS 188 -) is a newly defined biotin-binding site, which reduces the ability to bind to (strept)avidin family proteins. The novel streptavidin C1 could help in the development of an engineered tetrameric streptavidin with reduced biotin-binding capacity as well as other biomaterial tools.
Organizational Affiliation:
Department of Plant Biotechnology, School of Life Sciences and Biotechnology for BK21 PLUS, Institute of Life Science and Natural Resources, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul 02841, South Korea.