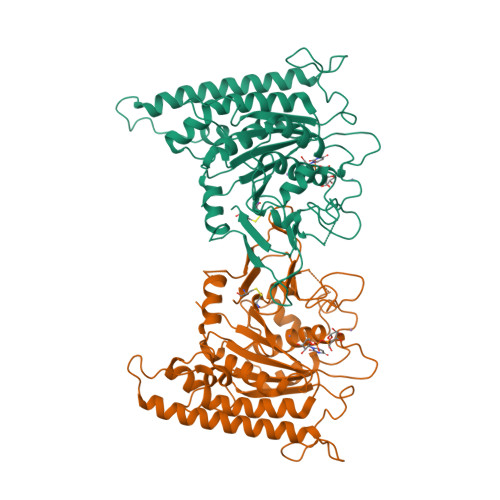
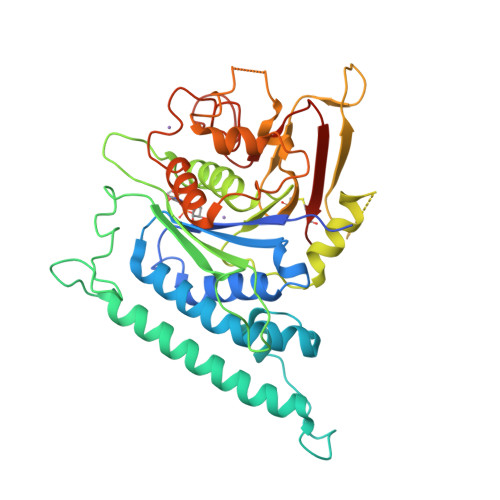
Structure of Arabidopsis CESA3 catalytic domain with its substrate UDP-glucose provides insight into the mechanism of cellulose synthesis.
Qiao, Z., Lampugnani, E.R., Yan, X.F., Khan, G.A., Saw, W.G., Hannah, P., Qian, F., Calabria, J., Miao, Y., Gruber, G., Persson, S., Gao, Y.G.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 33729990
- DOI: https://doi.org/10.1073/pnas.2024015118
- Primary Citation of Related Structures:
7CK1, 7CK2, 7CK3 - PubMed Abstract:
Cellulose is synthesized by cellulose synthases (CESAs) from the glycosyltransferase GT-2 family. In plants, the CESAs form a six-lobed rosette-shaped CESA complex (CSC). Here we report crystal structures of the catalytic domain of Arabidopsis thaliana CESA3 (AtCESA3 CatD ) in both apo and uridine diphosphate (UDP)-glucose (UDP-Glc)-bound forms. AtCESA3 CatD has an overall GT-A fold core domain sandwiched between a plant-conserved region (P-CR) and a class-specific region (C-SR). By superimposing the structure of AtCESA3 CatD onto the bacterial cellulose synthase BcsA, we found that the coordination of the UDP-Glc differs, indicating different substrate coordination during cellulose synthesis in plants and bacteria. Moreover, structural analyses revealed that AtCESA3 CatD can form a homodimer mainly via interactions between specific beta strands. We confirmed the importance of specific amino acids on these strands for homodimerization through yeast and in planta assays using point-mutated full-length AtCESA3. Our work provides molecular insights into how the substrate UDP-Glc is coordinated in the CESAs and how the CESAs might dimerize to eventually assemble into CSCs in plants.
Organizational Affiliation:
School of Biological Sciences, Nanyang Technological University, Singapore 637551.