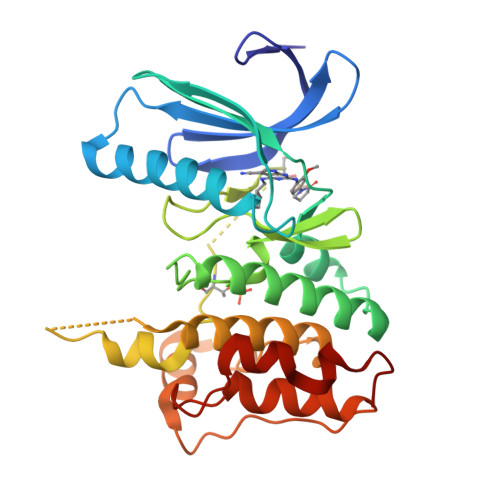
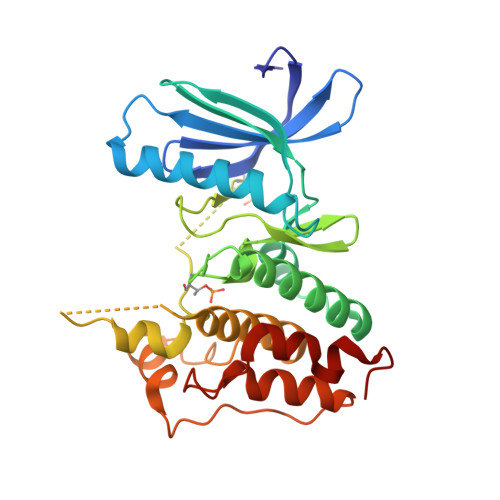
X-ray Crystal Structure-Guided Design and Optimization of 7 H -Pyrrolo[2,3- d ]pyrimidine-5-carbonitrile Scaffold as a Potent and Orally Active Monopolar Spindle 1 Inhibitor.
Lee, Y., Kim, H., Kim, H., Cho, H.Y., Jee, J.G., Seo, K.A., Son, J.B., Ko, E., Choi, H.G., Kim, N.D., Kim, I.(2021) J Med Chem 64: 6985-6995
- PubMed: 33942608
- DOI: https://doi.org/10.1021/acs.jmedchem.1c00542
- Primary Citation of Related Structures:
7CHM, 7CHN, 7CHT, 7CIL, 7CJA, 7CLH - PubMed Abstract:
Triple-negative breast cancer (TNBC) is an aggressive breast-cancer subtype associated with poor prognosis and high relapse rates. Monopolar spindle 1 kinase (MPS1) is an apical dual-specificity protein kinase that is over-expressed in TNBC. We herein report a highly selective MPS1 inhibitor based on a 7 H -pyrrolo[2,3- d ]pyrimidine-5-carbonitrile scaffold. Our lead optimization was guided by key X-ray crystal structure analysis. In vivo evaluation of candidate ( 9 ) is shown to effectively mitigate human TNBC cell proliferation.
Organizational Affiliation:
College of Pharmacy and Yonsei Institute of Pharmaceutical Sciences, Yonsei University, 85 Songdogwahak-ro, Yeonsu-gu, Incheon 21983, South Korea.