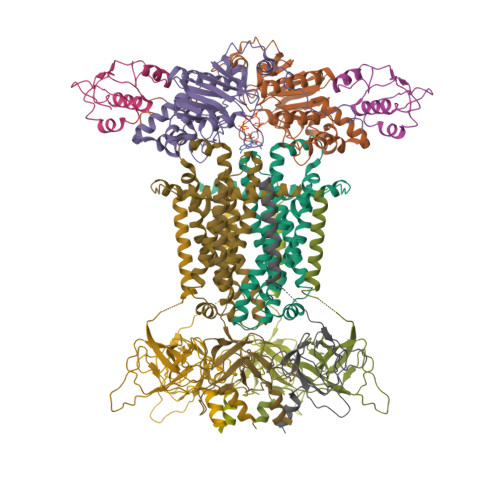
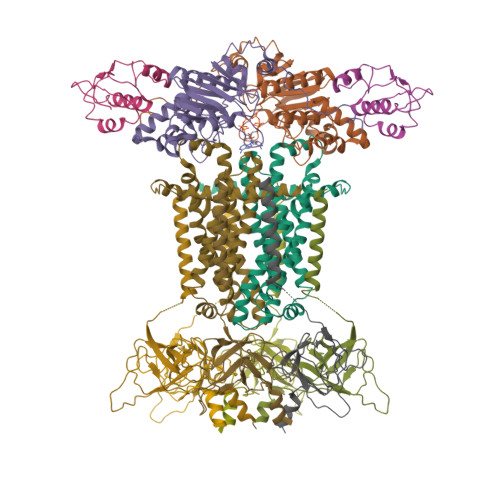
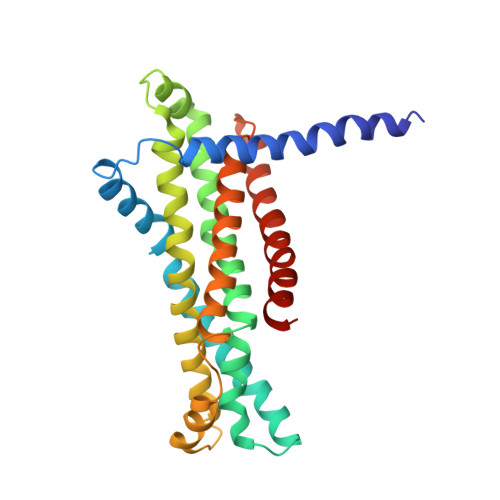
Structural mechanism of phospholipids translocation by MlaFEDB complex.
Chi, X., Fan, Q., Zhang, Y., Liang, K., Wan, L., Zhou, Q., Li, Y.(2020) Cell Res 30: 1127-1135
- PubMed: 32884137
- DOI: https://doi.org/10.1038/s41422-020-00404-6
- Primary Citation of Related Structures:
7CGE, 7CGN, 7CH0 - PubMed Abstract:
In Gram-negative bacteria, phospholipids are major components of the inner membrane and the inner leaflet of the outer membrane, playing an essential role in forming the unique dual-membrane barrier to exclude the entry of most antibiotics. Understanding the mechanisms of phospholipid translocation between the inner and outer membrane represents one of the major challenges surrounding bacterial phospholipid homeostasis. The conserved MlaFEDB complex in the inner membrane functions as an ABC transporter to drive the translocation of phospholipids between the inner membrane and the periplasmic protein MlaC. However, the mechanism of phospholipid translocation remains elusive. Here we determined three cryo-EM structures of MlaFEDB from Escherichia coli in its nucleotide-free and ATP-bound conformations, and performed extensive functional studies to verify and extend our findings from structural analyses. Our work reveals unique structural features of the entire MlaFEDB complex, six well-resolved phospholipids in three distinct cavities, and large-scale conformational changes upon ATP binding. Together, these findings define the cycle of structural rearrangement of MlaFEDB in action, and suggest that MlaFEDB uses an extrusion mechanism to extract and release phospholipids through the central translocation cavity.
Organizational Affiliation:
Center for Infectious Disease Research, Zhejiang Provincial Laboratory of Life Sciences and Biomedicine, Key Laboratory of Structural Biology of Zhejiang Province, School of Life Sciences, Westlake University, Hangzhou, Zhejiang, 310024, China.