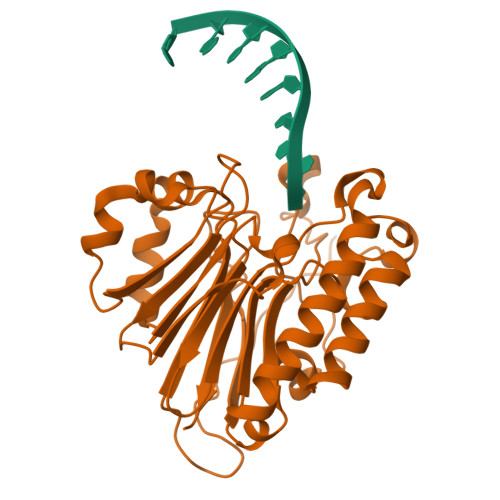
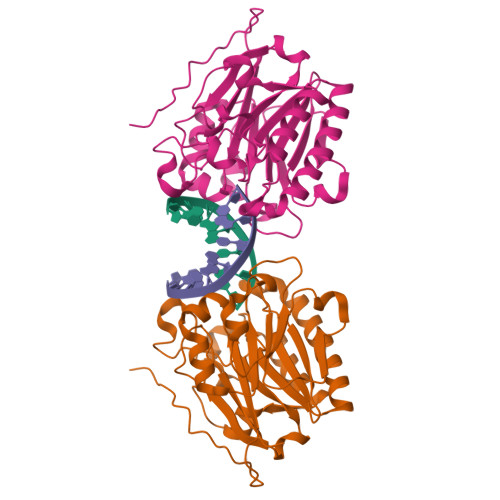
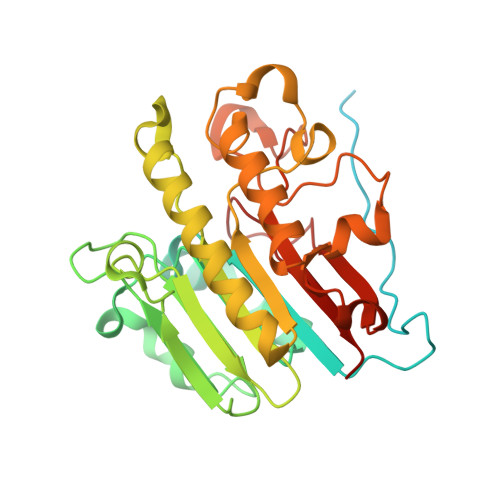
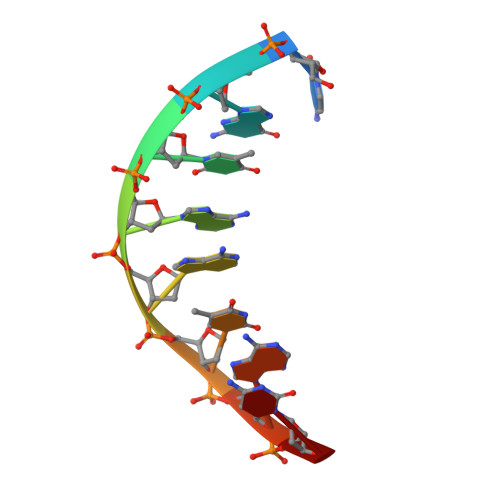
APE1 distinguishes DNA substrates in exonucleolytic cleavage by induced space-filling.
Liu, T.C., Lin, C.T., Chang, K.C., Guo, K.W., Wang, S., Chu, J.W., Hsiao, Y.Y.(2021) Nat Commun 12: 601-601
- PubMed: 33504804
- DOI: https://doi.org/10.1038/s41467-020-20853-2
- Primary Citation of Related Structures:
7CD5, 7CD6 - PubMed Abstract:
The exonuclease activity of Apurinic/apyrimidinic endonuclease 1 (APE1) is responsible for processing matched/mismatched terminus in various DNA repair pathways and for removing nucleoside analogs associated with drug resistance. To fill in the gap of structural basis for exonucleolytic cleavage, we determine the APE1-dsDNA complex structures displaying end-binding. As an exonuclease, APE1 does not show base preference but can distinguish dsDNAs with different structural features. Integration with assaying enzyme activity and binding affinity for a variety of substrates reveals for the first time that both endonucleolytic and exonucleolytic cleavage can be understood by an induced space-filling model. Binding dsDNA induces RM (Arg176 and Met269) bridge that defines a long and narrow product pocket for exquisite machinery of substrate selection. Our study paves the way to comprehend end-processing of dsDNA in the cell and the drug resistance relating to APE1.
Organizational Affiliation:
Institute of Molecular Medicine and Bioengineering, National Chiao Tung University, Hsinchu, 30068, Taiwan.