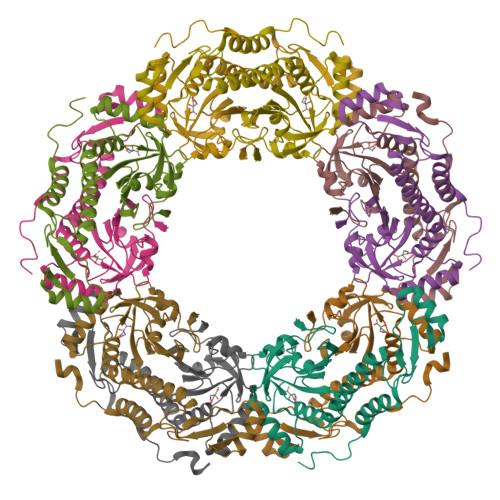
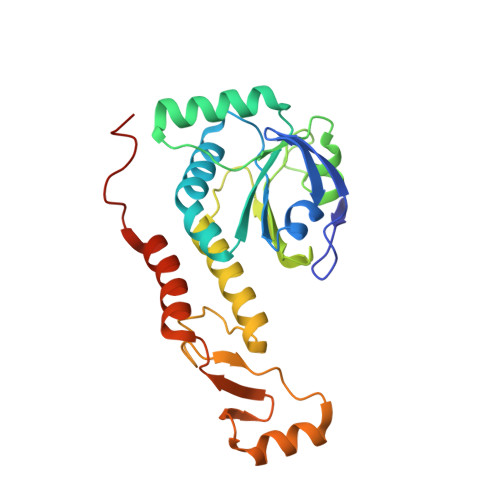
Rebuilding Ring-Type Assembly of Peroxiredoxin by Chemical Modification.
Himiyama, T., Tsuchiya, Y., Yonezawa, Y., Nakamura, T.(2021) Bioconjug Chem 32: 153-160
- PubMed: 33334100
- DOI: https://doi.org/10.1021/acs.bioconjchem.0c00587
- Primary Citation of Related Structures:
7C87, 7C89, 7C8A, 7CQJ - PubMed Abstract:
Direct control of the protein quaternary structure (QS) is challenging owing to the complexity of the protein structure. As a protein with a characteristic QS, peroxiredoxin from Aeropyrum pernix K1 (ApPrx) forms a decamer, wherein five dimers associate to form a ring. Here, we disrupted and reconstituted ApPrx QS via amino acid mutations and chemical modifications targeting hot spots for protein assembly. The decameric QS of an ApPrx* mutant, wherein all cysteine residues in wild-type ApPrx were mutated to serine, was destructed to dimers via an F80C mutation. The dimeric ApPrx*F80C mutant was then modified with a small molecule and successfully assembled as a decamer. Structural analysis confirmed that an artificially installed chemical moiety potentially facilitates suitable protein-protein interactions to rebuild a native structure. Rebuilding of dodecamer was also achieved through an additional amino acid mutation. This study describes a facile method to regulate the protein assembly state.
Organizational Affiliation:
Biomedical Research Institute, National Institute of Advanced Industrial Science and Technology, Ikeda, Osaka 563-8577, Japan.