Development of Novel AKR1C3 Inhibitors as New Potential Treatment for Castration-Resistant Prostate Cancer.
Endo, S., Oguri, H., Segawa, J., Kawai, M., Hu, D., Xia, S., Okada, T., Irie, K., Fujii, S., Gouda, H., Iguchi, K., Matsukawa, T., Fujimoto, N., Nakayama, T., Toyooka, N., Matsunaga, T., Ikari, A.(2020) J Med Chem 63: 10396-10411
- PubMed: 32847363
- DOI: https://doi.org/10.1021/acs.jmedchem.0c00939
- Primary Citation of Related Structures:
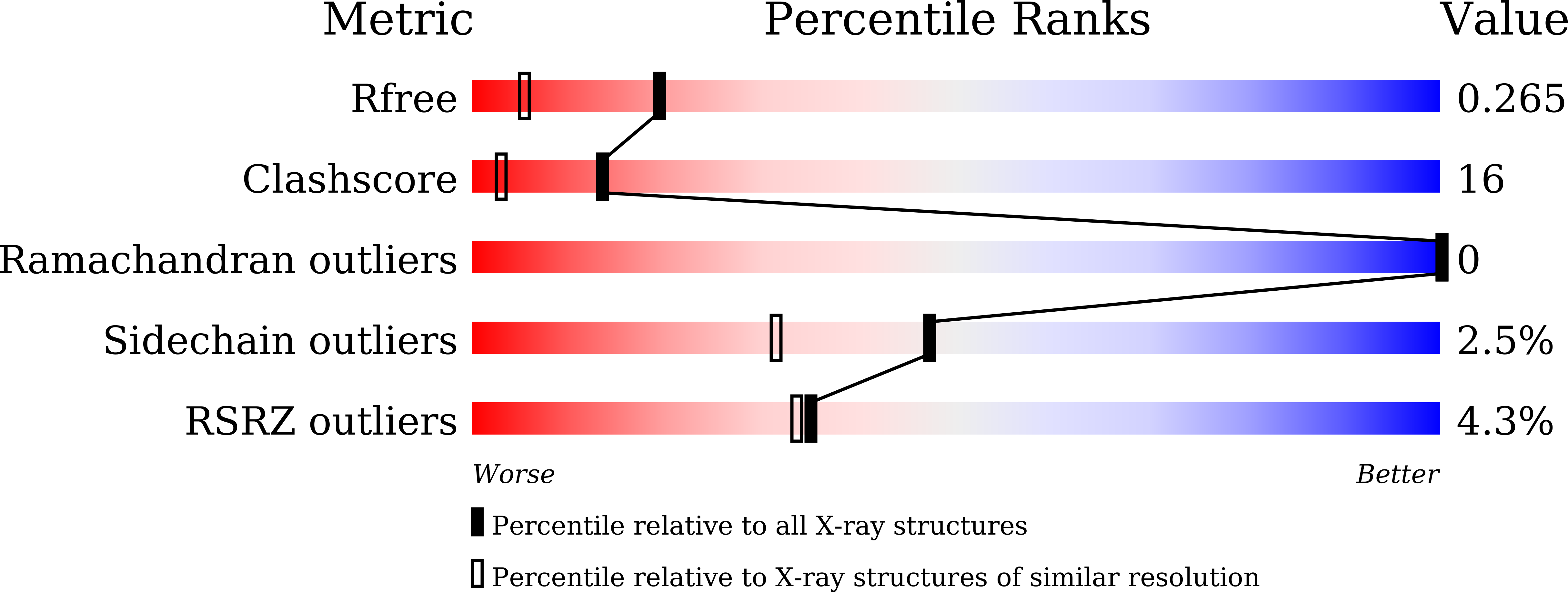
7C7F, 7C7G, 7C7H - PubMed Abstract:
Aldo-keto reductase (AKR) 1C3 catalyzes the synthesis of active androgens that promote the progression of prostate cancer. AKR1C3 also contributes to androgen-independent cell proliferation and survival through the metabolism of prostaglandins and reactive aldehydes. Because of its elevation in castration-resistant prostate cancer (CRPC) tissues, AKR1C3 is a promising therapeutic target for CRPC. In this study, we found a novel potent AKR1C3 inhibitor, N -(4-fluorophenyl)-8-hydroxy-2-imino-2 H -chromene-3-carboxamide ( 2d ), and synthesized its derivatives with IC 50 values of 25-56 nM and >220-fold selectivity over other AKRs (1C1, 1C2, and 1C4). The structural factors for the inhibitory potency were elucidated by crystallographic study of AKR1C3 complexes with 2j and 2l . The inhibitors suppressed proliferation of prostate cancer 22Rv1 and PC3 cells through both androgen-dependent and androgen-independent mechanisms. Additionally, 2j and 2l prevented prostate tumor growth in a xenograft mouse model. Furthermore, the inhibitors significantly augmented apoptotic cell death induced by anti-CRPC drugs (abiraterone or enzalutamide).
Organizational Affiliation:
Laboratory of Biochemistry, Gifu Pharmaceutical University, Gifu 501-1196, Japan.