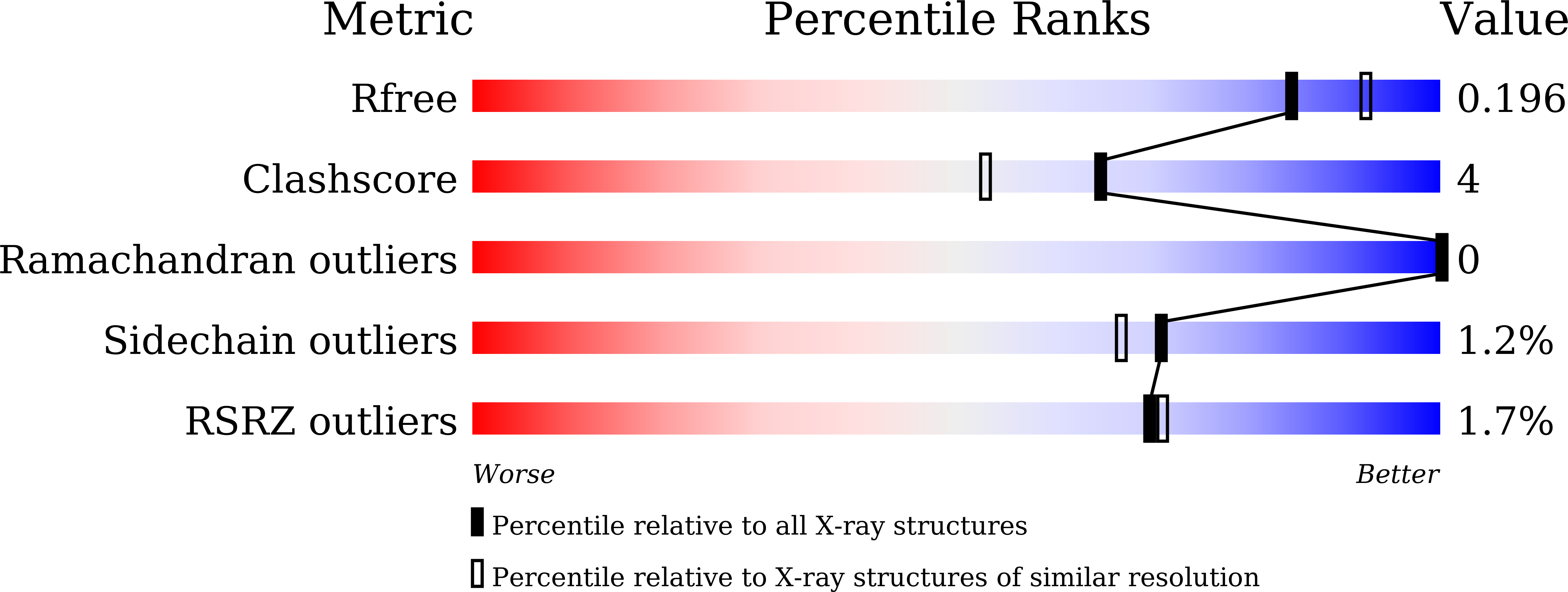
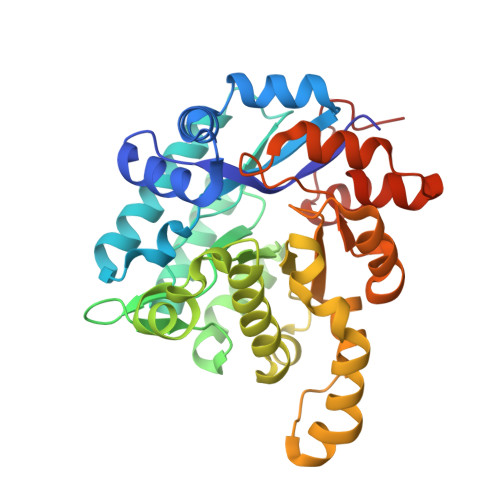
2,3-Dihydroxybenzoic Acid Decarboxylase from Fusarium oxysporum: Crystal Structures and Substrate Recognition Mechanism.
Song, M., Zhang, X., Liu, W., Feng, J., Cui, Y., Yao, P., Wang, M., Guo, R.T., Wu, Q., Zhu, D.(2020) Chembiochem 21: 2950-2956
- PubMed: 32421914
- DOI: https://doi.org/10.1002/cbic.202000244
- Primary Citation of Related Structures:
6M53, 7BP1, 7BPC - PubMed Abstract:
A 2,3-dihydroxybenzoic acid decarboxylase from Fusarium oxysporum (2,3-DHBD_Fo) has a relatively high catalytic efficiency for the decarboxylation of 2,3-dihydroxybenzoic acid (DHBA) and carboxylation of catechol, thus it has a different substrate spectrum from other benzoic acid decarboxylases. We have determined the structures of 2,3-DHBD_Fo in its apo form and complexes with catechol or 2,5-dihydroxybenzoic acid at 1.55, 1.97, and 2.45 Å resolution, respectively. The crystal structures of 2,3-DHBD_Fo show that the enzyme exists as a homotetramer, and each active center has a Zn 2+ ion coordinated by E8, H167, D291 and three water molecules. This is different from 2,6-DHBD from Rhizobium sporomusa, in which the Zn 2+ ion is also coordinated with H10. Surprisingly, mutation of A10 of 2,3-DHBD_Fo to His resulted in almost complete loss of the enzyme activity. Enzyme-substrate docking and site-directed mutation studies indicate that residue R233 Δ interacts with the 3-hydroxy group of 2,3-DHBA, and plays an important role in substrate recognition for this enzyme, thus revealing the molecular basis 2,3-dihydroxybenzoic acid decarboxylase.
Organizational Affiliation:
Key Laboratory of Industrial Fermentation Microbiology, Tianjin University of Science and Technology, Tianjin, 300457, P. R. China.