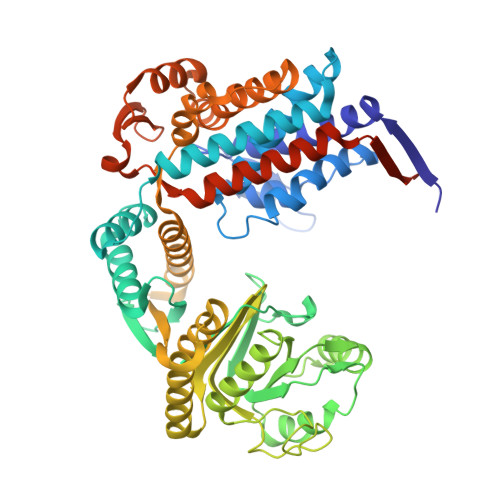
Cryo-EM structure of human mitochondrial HSPD1.
Klebl, D.P., Feasey, M.C., Hesketh, E.L., Ranson, N.A., Wurdak, H., Sobott, F., Bon, R.S., Muench, S.P.(2021) iScience 24: 102022-102022
- PubMed: 33506187
- DOI: https://doi.org/10.1016/j.isci.2020.102022
- Primary Citation of Related Structures:
7AZP - PubMed Abstract:
Chaperonins play an important role in folding newly synthesized or translocated proteins in all organisms. The bacterial chaperonin GroEL has served as a model system for the understanding of these proteins. In comparison, its human homolog, known as mitochondrial heat shock protein family member D1 (HSPD1) is poorly understood. Here, we present the structure of HSPD1 in the apo state determined by cryo-electron microscopy (cryo-EM). Unlike GroEL, HSPD1 forms mostly single ring assemblies in the absence of co-chaperonin (HSPE1). Comparison with GroEL shows a rotation and increased flexibility of the apical domain. Together with published structures of the HSPD1/HSPE1 co-chaperonin complex, this work gives insight into the structural changes that occur during the catalytic cycle. This new understanding of HSPD1 structure and its rearrangements upon complex formation may provide new insights for the development of HSPD1-targeting treatments against a diverse range of diseases including glioblastoma.
Organizational Affiliation:
School of Biomedical Sciences, Faculty of Biological Sciences & Astbury Centre for Structural and Molecular Biology, University of Leeds, Leeds LS2 9JT, UK.