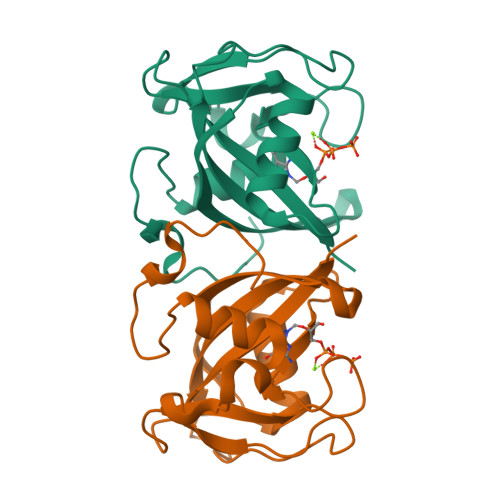
NUDT15-mediated hydrolysis limits the efficacy of anti-HCMV drug ganciclovir.
Zhang, S.M., Rehling, D., Jemth, A.S., Throup, A., Landazuri, N., Almlof, I., Gottmann, M., Valerie, N.C.K., Borhade, S.R., Wakchaure, P., Page, B.D.G., Desroses, M., Homan, E.J., Scobie, M., Rudd, S.G., Berglund, U.W., Soderberg-Naucler, C., Stenmark, P., Helleday, T.(2021) Cell Chem Biol 28: 1693-1702.e6
- PubMed: 34192523
- DOI: https://doi.org/10.1016/j.chembiol.2021.06.001
- Primary Citation of Related Structures:
7AOM, 7AOP - PubMed Abstract:
Ganciclovir (GCV) is the first-line therapy against human cytomegalovirus (HCMV), a widespread infection that is particularly dangerous for immunodeficient individuals. Closely resembling deoxyguanosine triphosphate, the tri-phosphorylated metabolite of GCV (GCV-TP) is preferentially incorporated by the viral DNA polymerase, thereby terminating chain extension and, eventually, viral replication. However, the treatment outcome of GCV varies greatly among individuals, therefore warranting better understanding of its metabolism. Here we show that NUDT15, a Nudix hydrolase known to metabolize thiopurine triphosphates, can similarly hydrolyze GCV-TP through biochemical studies and co-crystallization of the NUDT15/GCV-TP complex. More critically, GCV efficacy was potentiated in HCMV-infected cells following NUDT15 depletion by RNAi or inhibition by an in-house-developed, nanomolar NUDT15 inhibitor, TH8321, suggesting that pharmacological targeting of NUDT15 is a possible avenue to improve existing anti-HCMV regimens. Collectively, the data further implicate NUDT15 as a broad-spectrum metabolic regulator of nucleoside analog therapeutics, such as thiopurines and GCV.
Organizational Affiliation:
Science for Life Laboratory, Department of Oncology-Pathology, Karolinska Institutet, Box 1031, 17165 Stockholm, Sweden.