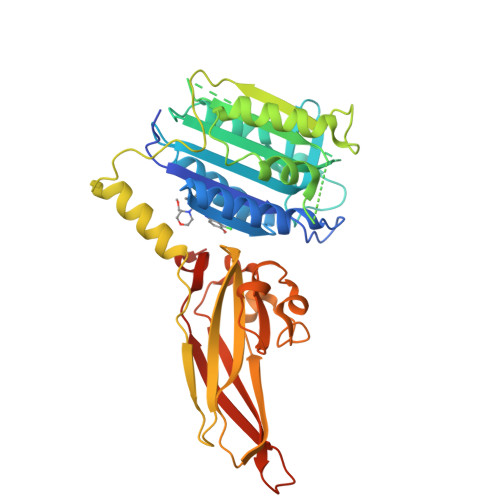
Discovery of Potent, Highly Selective, and In Vivo Efficacious, Allosteric MALT1 Inhibitors by Iterative Scaffold Morphing.
Pissot Soldermann, C., Simic, O., Renatus, M., Erbel, P., Melkko, S., Wartmann, M., Bigaud, M., Weiss, A., McSheehy, P., Endres, R., Santos, P., Blank, J., Schuffenhauer, A., Bold, G., Buschmann, N., Zoller, T., Altmann, E., Manley, P.W., Dix, I., Buchdunger, E., Scesa, J., Quancard, J., Schlapbach, A., Bornancin, F., Radimerski, T., Regnier, C.H.(2020) J Med Chem 63: 14576-14593
- PubMed: 33252239
- DOI: https://doi.org/10.1021/acs.jmedchem.0c01245
- Primary Citation of Related Structures:
7AK0, 7AK1 - PubMed Abstract:
MALT1 plays a central role in immune cell activation by transducing NF-κB signaling, and its proteolytic activity represents a key node for therapeutic intervention. Two cycles of scaffold morphing of a high-throughput biochemical screening hit resulted in the discovery of MLT-231, which enabled the successful pharmacological validation of MALT1 allosteric inhibition in preclinical models of humoral immune responses and B-cell lymphomas. Herein, we report the structural activity relationships (SARs) and analysis of the physicochemical properties of a pyrazolopyrimidine-derived compound series. In human T-cells and B-cell lymphoma lines, MLT-231 potently and selectively inhibits the proteolytic activity of MALT1 in NF-κB-dependent assays. Both in vitro and in vivo profiling of MLT-231 support further optimization of this in vivo tool compound toward preclinical characterization.
Organizational Affiliation:
Novartis Institutes for BioMedical Research, Novartis Campus, CH-4002 Basel, Switzerland.