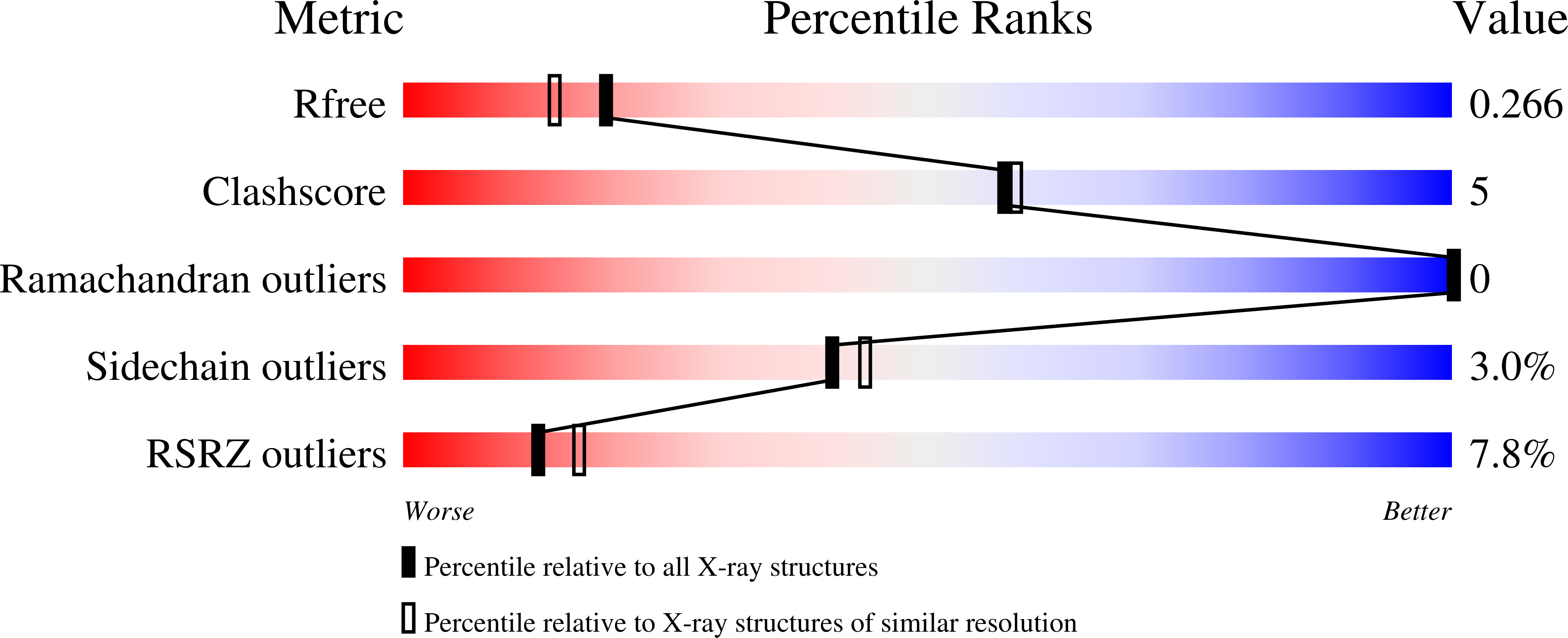
Structural and Biochemical Investigation of Class I Ribonucleotide Reductase from the Hyperthermophile Aquifex aeolicus.
Rehling, D., Scaletti, E.R., Rozman Grinberg, I., Lundin, D., Sahlin, M., Hofer, A., Sjoberg, B.M., Stenmark, P.(2022) Biochemistry 61: 92-106
- PubMed: 34941255
- DOI: https://doi.org/10.1021/acs.biochem.1c00503
- Primary Citation of Related Structures:
7AGJ, 7AIK, 7AIL, 7Q39, 7Q3C - PubMed Abstract:
Ribonucleotide reductase (RNR) is an essential enzyme with a complex mechanism of allosteric regulation found in nearly all living organisms. Class I RNRs are composed of two proteins, a large α-subunit (R1) and a smaller β-subunit (R2) that exist as homodimers, that combine to form an active heterotetramer. Aquifex aeolicus is a hyperthermophilic bacterium with an unusual RNR encoding a 346-residue intein in the DNA sequence encoding its R2 subunit. We present the first structures of the A. aeolicus R1 and R2 (AaR1 and AaR2, respectively) proteins as well as the biophysical and biochemical characterization of active and inactive A. aeolicus RNR. While the active oligomeric state and activity regulation of A. aeolicus RNR are similar to those of other characterized RNRs, the X-ray crystal structures also reveal distinct features and adaptations. Specifically, AaR1 contains a β-hairpin hook structure at the dimer interface, which has an interesting π-stacking interaction absent in other members of the NrdAh subclass, and its ATP cone houses two ATP molecules. We determined structures of two AaR2 proteins: one purified from a construct lacking the intein (AaR2) and a second purified from a construct including the intein sequence (AaR2_genomic). These structures in the context of metal content analysis and activity data indicate that AaR2_genomic displays much higher iron occupancy and activity compared to AaR2, suggesting that the intein is important for facilitating complete iron incorporation, particularly in the Fe2 site of the mature R2 protein, which may be important for the survival of A. aeolicus in low-oxygen environments.
Organizational Affiliation:
Department of Biochemistry and Biophysics, Stockholm University, S-106 91 Stockholm, Sweden.