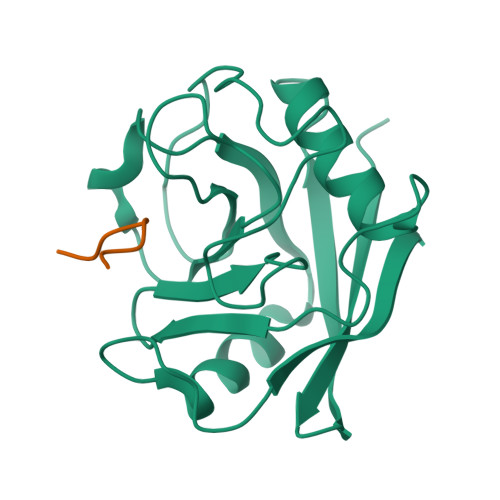
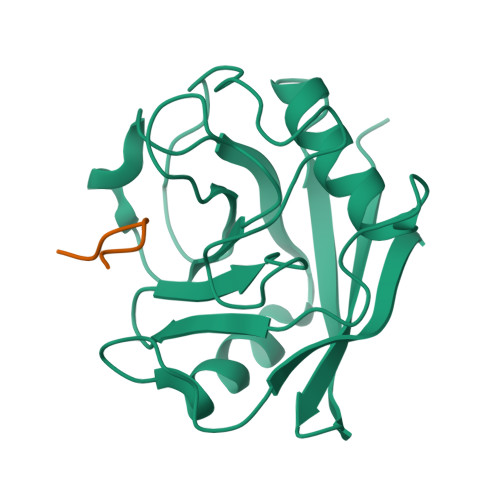
Proline/arginine dipeptide repeat polymers derail protein folding in amyotrophic lateral sclerosis.
Babu, M., Favretto, F., de Opakua, A.I., Rankovic, M., Becker, S., Zweckstetter, M.(2021) Nat Commun 12: 3396-3396
- PubMed: 34099711
- DOI: https://doi.org/10.1038/s41467-021-23691-y
- Primary Citation of Related Structures:
7ABT - PubMed Abstract:
Amyotrophic lateral sclerosis and frontotemporal dementia are two neurodegenerative diseases with overlapping clinical features and the pathological hallmark of cytoplasmic deposits of misfolded proteins. The most frequent cause of familial forms of these diseases is a hexanucleotide repeat expansion in the non-coding region of the C9ORF72 gene that is translated into dipeptide repeat polymers. Here we show that proline/arginine repeat polymers derail protein folding by sequestering molecular chaperones. We demonstrate that proline/arginine repeat polymers inhibit the folding catalyst activity of PPIA, an abundant molecular chaperone and prolyl isomerase in the brain that is altered in amyotrophic lateral sclerosis. NMR spectroscopy reveals that proline/arginine repeat polymers bind to the active site of PPIA. X-ray crystallography determines the atomic structure of a proline/arginine repeat polymer in complex with the prolyl isomerase and defines the molecular basis for the specificity of disease-associated proline/arginine polymer interactions. The combined data establish a toxic mechanism that is specific for proline/arginine dipeptide repeat polymers and leads to derailed protein homeostasis in C9orf72-associated neurodegenerative diseases.
Organizational Affiliation:
German Center for Neurodegenerative Diseases (DZNE), Göttingen, Germany.