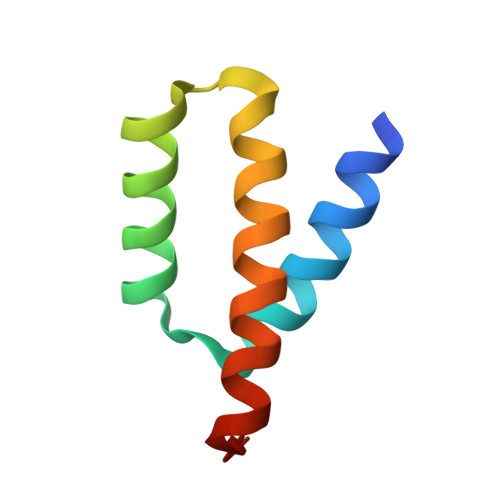
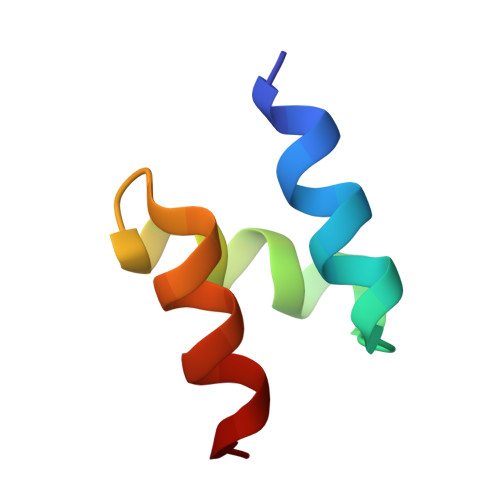Structure of HIV-1 Vpr in complex with the human nucleotide excision repair protein hHR23A.
Byeon, I.L., Calero, G., Wu, Y., Byeon, C.H., Jung, J., DeLucia, M., Zhou, X., Weiss, S., Ahn, J., Hao, C., Skowronski, J., Gronenborn, A.M.(2021) Nat Commun 12: 6864-6864
- PubMed: 34824204
- DOI: https://doi.org/10.1038/s41467-021-27009-w
- Primary Citation of Related Structures:
6XQI, 6XQJ - PubMed Abstract:
HIV-1 Vpr is a prototypic member of a large family of structurally related lentiviral virulence factors that antagonize various aspects of innate antiviral immunity. It subverts host cell DNA repair and protein degradation machineries by binding and inhibiting specific post-replication repair enzymes, linking them via the DCAF1 substrate adaptor to the Cullin 4 RING E3 ligase (CRL4 DCAF1 ). HIV-1 Vpr also binds to the multi-domain protein hHR23A, which interacts with the nucleotide excision repair protein XPC and shuttles ubiquitinated proteins to the proteasome. Here, we report the atomic resolution structure of Vpr in complex with the C-terminal half of hHR23A, containing the XPC-binding (XPCB) and ubiquitin-associated (UBA2) domains. The XPCB and UBA2 domains bind to different sides of Vpr's 3-helix-bundle structure, with UBA2 interacting with the α2 and α3 helices of Vpr, while the XPCB domain contacts the opposite side of Vpr's α3 helix. The structure as well as biochemical results reveal that hHR23A and DCAF1 use overlapping binding surfaces on Vpr, even though the two proteins exhibit entirely different three-dimensional structures. Our findings show that Vpr independently targets hHR23A- and DCAF1- dependent pathways and highlight HIV-1 Vpr as a versatile module that interferes with DNA repair and protein degradation pathways.
- Pittsburgh Center for HIV Protein Interactions, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Organizational Affiliation: