Functional implications of MIR domains in protein O -mannosylation.
Chiapparino, A., Grbavac, A., Jonker, H.R., Hackmann, Y., Mortensen, S., Zatorska, E., Schott, A., Stier, G., Saxena, K., Wild, K., Schwalbe, H., Strahl, S., Sinning, I.(2020) Elife 9
- PubMed: 33357379
- DOI: https://doi.org/10.7554/eLife.61189
- Primary Citation of Related Structures:
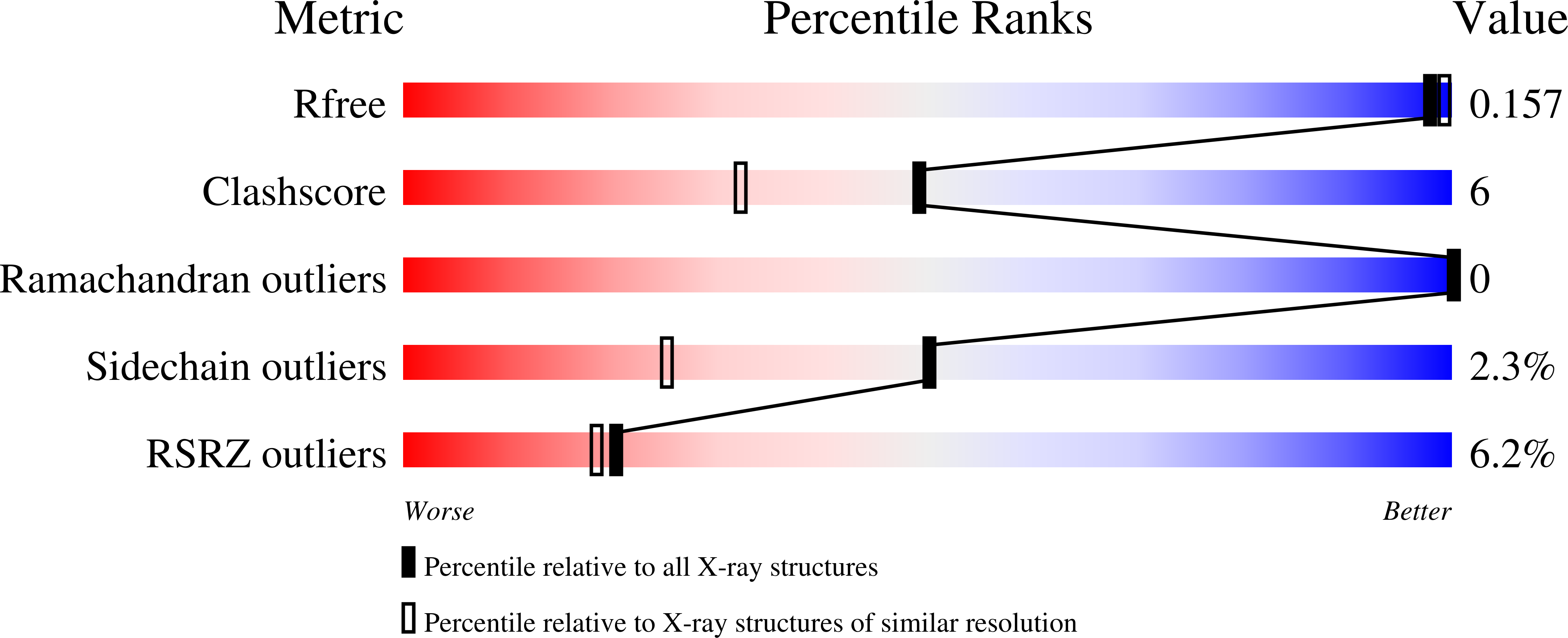
6ZQP, 6ZQQ - PubMed Abstract:
Protein O -mannosyltransferases (PMTs) represent a conserved family of multispanning endoplasmic reticulum membrane proteins involved in glycosylation of S/T-rich protein substrates and unfolded proteins. PMTs work as dimers and contain a luminal MIR domain with a β-trefoil fold, which is susceptive for missense mutations causing α-dystroglycanopathies in humans. Here, we analyze PMT-MIR domains by an integrated structural biology approach using X-ray crystallography and NMR spectroscopy and evaluate their role in PMT function in vivo. We determine Pmt2- and Pmt3-MIR domain structures and identify two conserved mannose-binding sites, which are consistent with general β-trefoil carbohydrate-binding sites (α, β), and also a unique PMT2-subfamily exposed FKR motif. We show that conserved residues in site α influence enzyme processivity of the Pmt1-Pmt2 heterodimer in vivo. Integration of the data into the context of a Pmt1-Pmt2 structure and comparison with homologous β-trefoil - carbohydrate complexes allows for a functional description of MIR domains in protein O -mannosylation.
Organizational Affiliation:
Heidelberg University Biochemistry Center (BZH), Heidelberg, Germany.