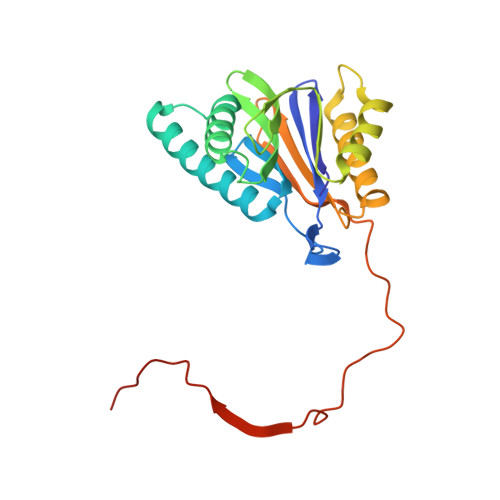
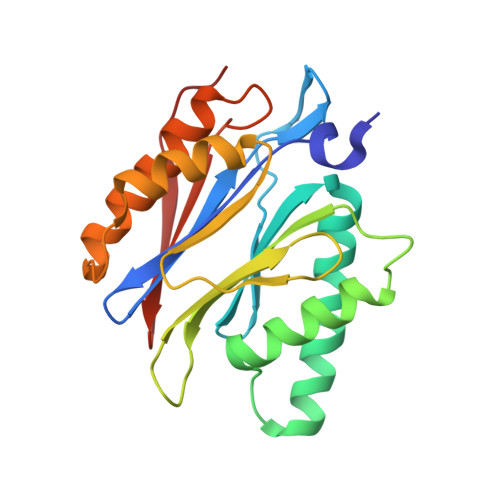
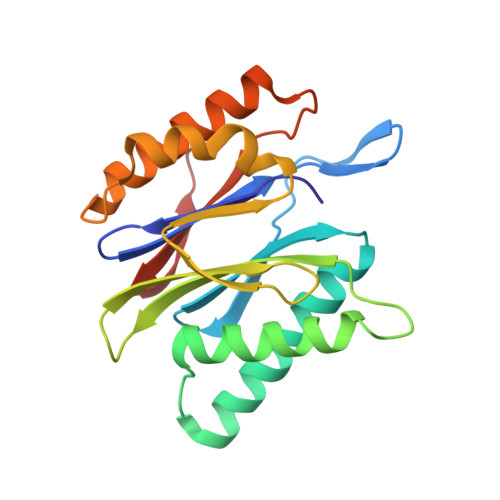
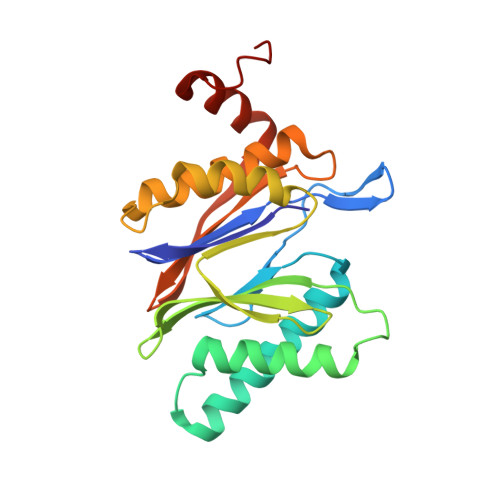
Activation, Structure, Biosynthesis and Bioactivity of Glidobactin-like Proteasome Inhibitors from Photorhabdus laumondii.
Zhao, L., Le Chapelain, C., Brachmann, A.O., Kaiser, M., Groll, M., Bode, H.B.(2021) Chembiochem 22: 1582-1588
- PubMed: 33452852
- DOI: https://doi.org/10.1002/cbic.202100014
- Primary Citation of Related Structures:
6ZOU, 6ZP6, 6ZP8 - PubMed Abstract:
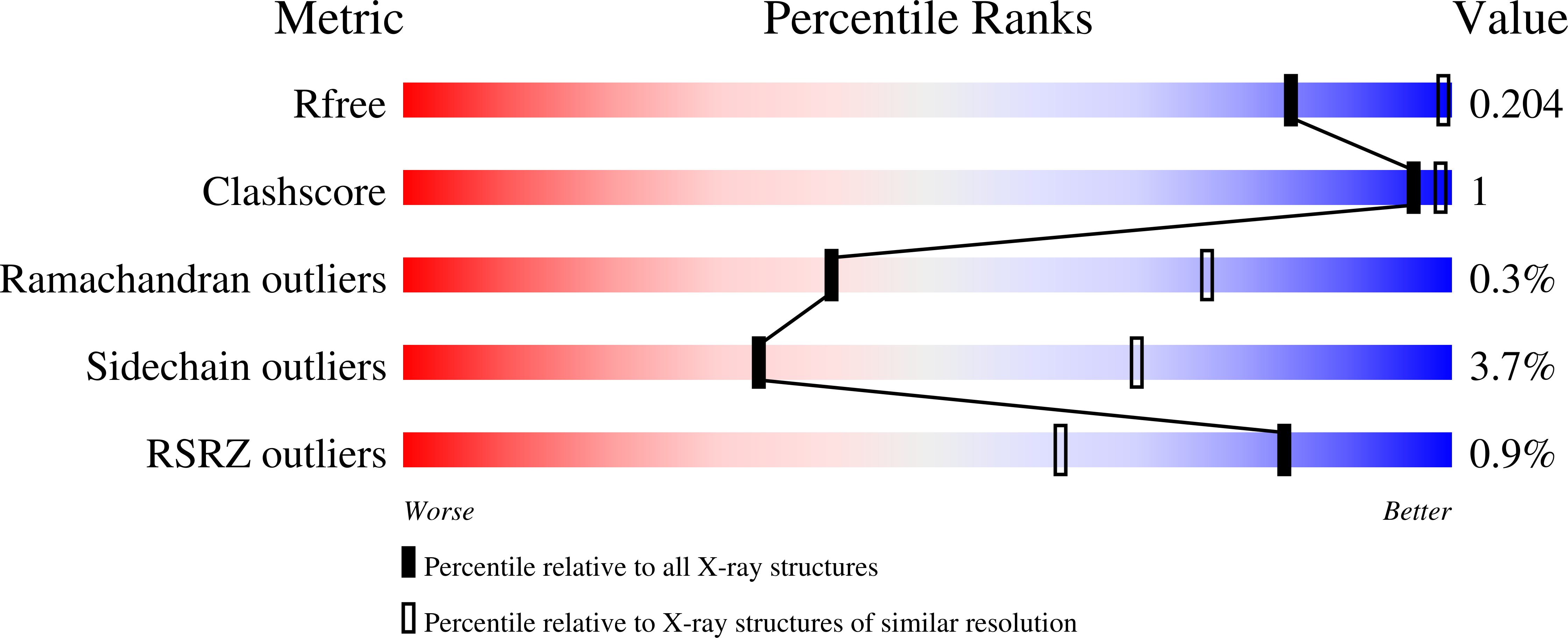
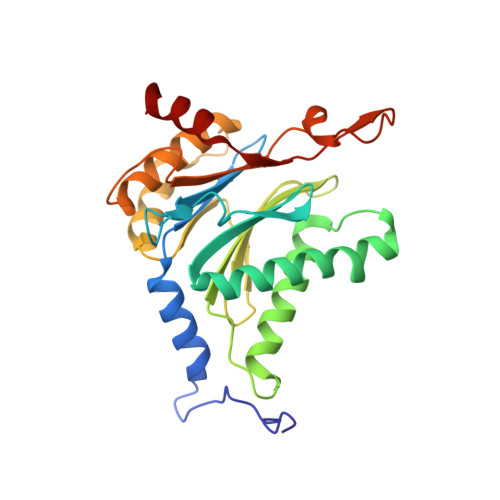
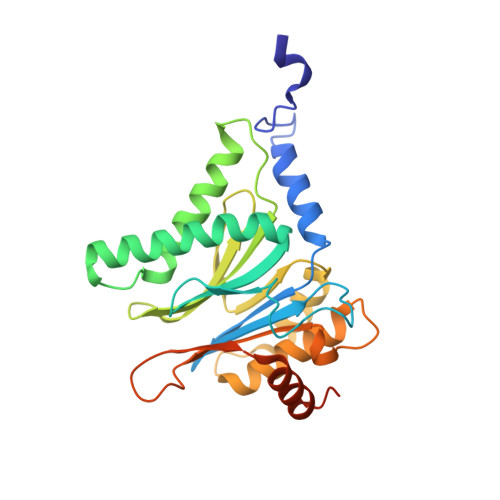
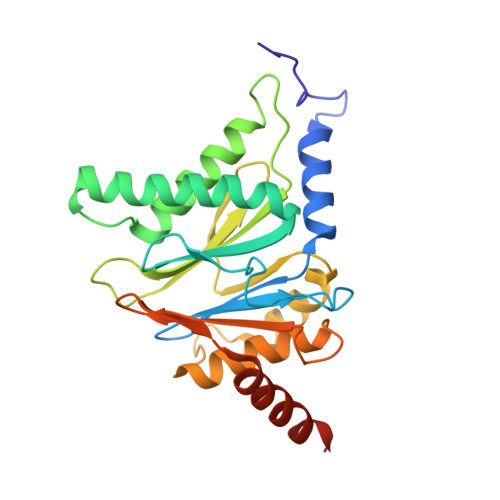
The glidobactin-like natural products (GLNPs) glidobactin A and cepafungin I have been reported to be potent proteasome inhibitors and are regarded as promising candidates for anticancer drug development. Their biosynthetic gene cluster (BGC) plu1881-1877 is present in entomopathogenic Photorhabdus laumondii but silent under standard laboratory conditions. Here we show the largest subset of GLNPs, which are produced and identified after activation of the silent BGC in the native host and following heterologous expression of the BGC in Escherichia coli. Their chemical diversity results from a relaxed substrate specificity and flexible product release in the assembly line of GLNPs. Crystal structure analysis of the yeast proteasome in complex with new GLNPs suggests that the degree of unsaturation and the length of the aliphatic tail are critical for their bioactivity. The results in this study provide the basis to engineer the BGC for the generation of new GLNPs and to optimize these natural products resulting in potential drugs for cancer therapy.
Organizational Affiliation:
Molecular Biotechnology, Department of Biosciences, Goethe University Frankfurt, 60438, Frankfurt am Main, Germany.