Deconstructing Noncovalent Kelch-like ECH-Associated Protein 1 (Keap1) Inhibitors into Fragments to Reconstruct New Potent Compounds.
Pallesen, J.S., Narayanan, D., Tran, K.T., Solbak, S.M.O., Marseglia, G., Sorensen, L.M.E., Hoj, L.J., Munafo, F., Carmona, R.M.C., Garcia, A.D., Desu, H.L., Brambilla, R., Johansen, T.N., Popowicz, G.M., Sattler, M., Gajhede, M., Bach, A.(2021) J Med Chem 64: 4623-4661
- PubMed: 33818106
- DOI: https://doi.org/10.1021/acs.jmedchem.0c02094
- Primary Citation of Related Structures:
6ZEW, 6ZEX, 6ZEY, 6ZEZ, 6ZF0, 6ZF1, 6ZF2, 6ZF3, 6ZF4, 6ZF5, 6ZF6, 6ZF7, 6ZF8 - PubMed Abstract:
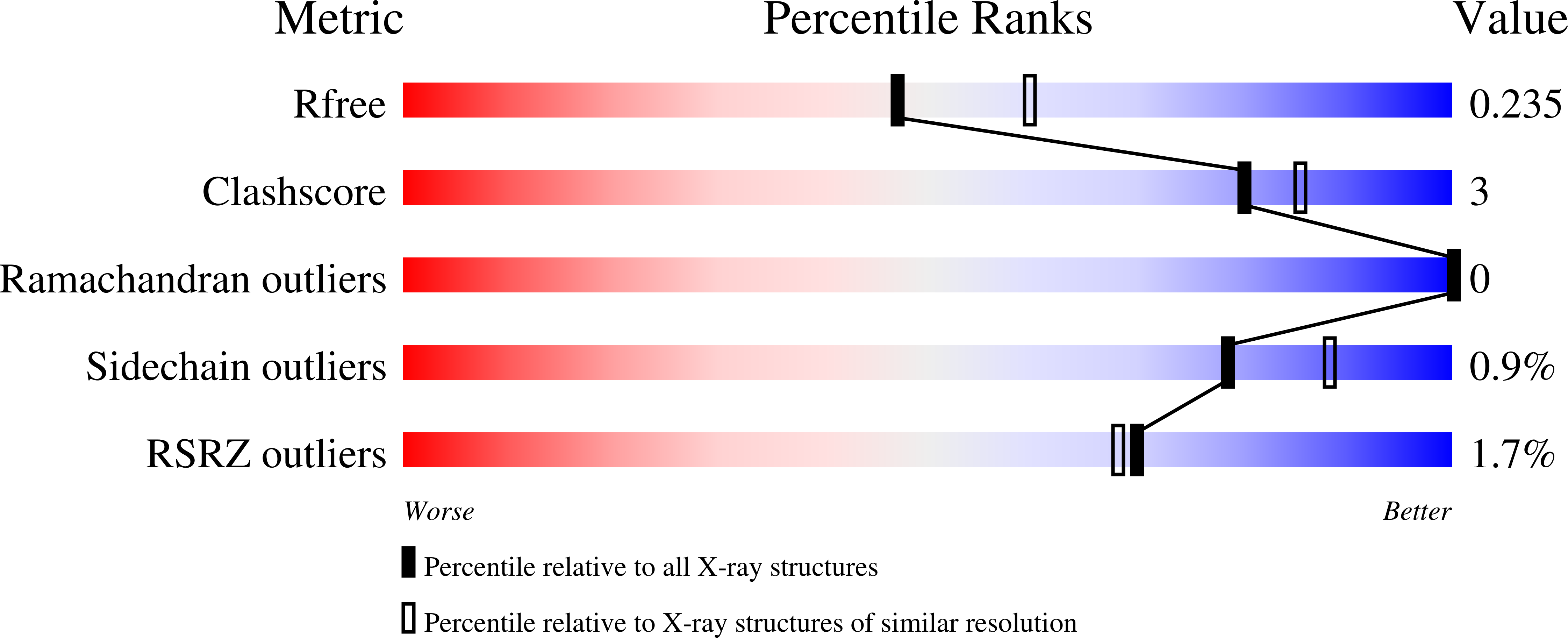
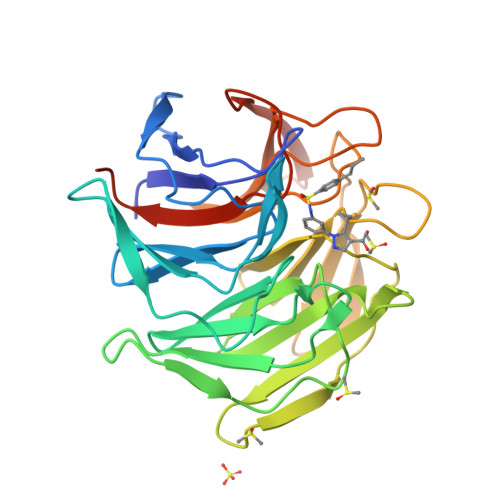
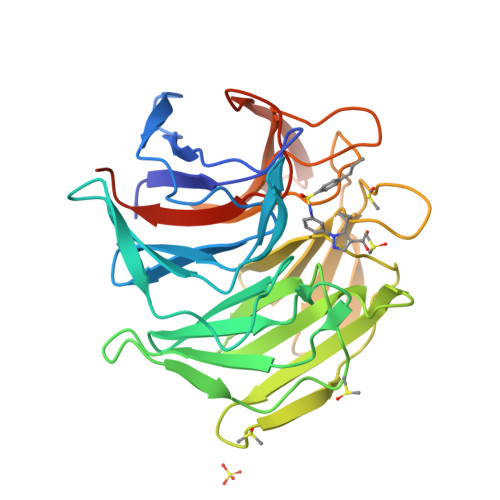
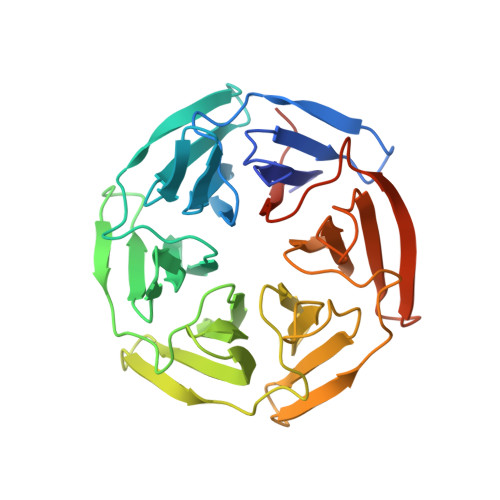
Targeting the protein-protein interaction (PPI) between nuclear factor erythroid 2-related factor 2 (Nrf2) and Kelch-like ECH-associated protein 1 (Keap1) is a potential therapeutic strategy to control diseases involving oxidative stress. Here, six classes of known small-molecule Keap1-Nrf2 PPI inhibitors were dissected into 77 fragments in a fragment-based deconstruction reconstruction (FBDR) study and tested in four orthogonal assays. This gave 17 fragment hits of which six were shown by X-ray crystallography to bind in the Keap1 Kelch binding pocket. Two hits were merged into compound 8 with a 220-380-fold stronger affinity ( K i = 16 μM) relative to the parent fragments. Systematic optimization resulted in several novel analogues with K i values of 0.04-0.5 μM, binding modes determined by X-ray crystallography, and enhanced microsomal stability. This demonstrates how FBDR can be used to find new fragment hits, elucidate important ligand-protein interactions, and identify new potent inhibitors of the Keap1-Nrf2 PPI.
Organizational Affiliation:
Department of Drug Design and Pharmacology, Faculty of Health and Medical Sciences, University of Copenhagen, Universitetsparken 2, DK-2100 Copenhagen, Denmark.