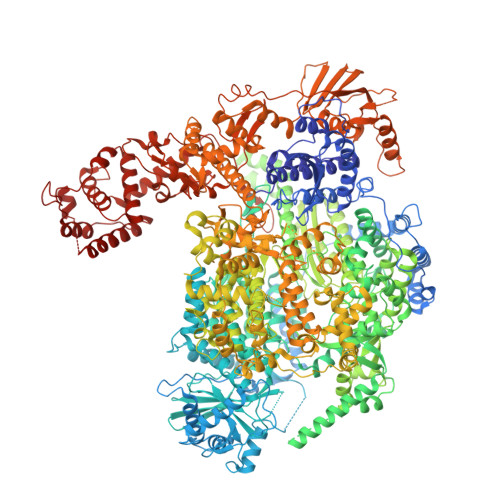
Pre-initiation and elongation structures of full-length La Crosse virus polymerase reveal functionally important conformational changes.
Arragain, B., Effantin, G., Gerlach, P., Reguera, J., Schoehn, G., Cusack, S., Malet, H.(2020) Nat Commun 11: 3590-3590
- PubMed: 32681014
- DOI: https://doi.org/10.1038/s41467-020-17349-4
- Primary Citation of Related Structures:
6Z6B, 6Z6G, 6Z8K - PubMed Abstract:
Bunyavirales is an order of segmented negative-strand RNA viruses comprising several life-threatening pathogens against which no effective treatment is currently available. Replication and transcription of the RNA genome constitute essential processes performed by the virally encoded multi-domain RNA-dependent RNA polymerase. Here, we describe the complete high-resolution cryo-EM structure of La Crosse virus polymerase. It reveals the presence of key protruding C-terminal domains, notably the cap-binding domain, which undergoes large movements related to its role in transcription initiation, and a zinc-binding domain that displays a fold not previously observed. We capture the polymerase structure at pre-initiation and elongation states, uncovering the coordinated movement of the priming loop, mid-thumb ring linker and lid domain required for the establishment of a ten-base-pair template-product RNA duplex before strand separation into respective exit tunnels. These structural details and the observed dynamics of key functional elements will be instrumental for structure-based development of polymerase inhibitors.
Organizational Affiliation:
Université Grenoble Alpes, CNRS, CEA, Institute for Structural Biology (IBS), F-38000, Grenoble, France.